Jed Quiaoit
Caroline Koffke
AP Biology 🧬
358 resourcesSee Units
All living systems require a constant input of energy to sustain life processes. The energy is used to power metabolic processes such as growth, repair, and reproduction, as well as to maintain the internal environment of the organism.
The primary source of energy for most living organisms is the food they consume, which is converted into a useable form of energy through metabolic pathways such as cellular respiration. Photosynthetic organisms, such as plants, can also produce their own energy through the process of photosynthesis, which converts light energy into chemical energy. 🌱
Meet Adenosine Triphosphate!
Most, if not all, energy originates from the sun, too! This energy is converted into cellular energy by photosynthetic organisms. This energy can then be broken down further into the most basic form of cellular energy, adenosine triphosphate or simply ATP. 🌞
In general, cellular processes are highly regulated, and energy-producing reactions are often paired with energy-consuming reactions. This happens all the time in the cell, and photosynthesis will be a great example of this as alluded to above.

Image courtesy of Giphy.
Order & Entropy
Life requires a highly ordered system, and the second law of thermodynamics is not violated in this process. The second law of thermodynamics states that entropy, or disorder, in a closed system will always increase over time. However, living organisms are open systems, constantly exchanging matter and energy with their environment, which allows them to maintain a high degree of order and organization.
One of the fundamental principles of life is that energy input must exceed energy loss in order to maintain order and power cellular processes. This is known as the principle of energy balance. To maintain this balance, living organisms have evolved metabolic pathways that are highly regulated and efficient, allowing them to transfer energy from one process to another with minimal loss.
Cellular processes that release energy, such as cellular respiration or fermentation, may be coupled with cellular processes that require energy, such as the synthesis of new molecules or the pumping of ions across a membrane. This allows organisms to efficiently transfer energy from one process to another, minimizing losses and maximizing gains.
However, when the energy input no longer exceeds the energy loss, the organism is no longer able to maintain its highly ordered state and its life processes. This results in death, as the organism is no longer able to carry out the metabolic processes necessary to maintain life. This is why the constant input of energy is crucial for the survival of all living systems! 💀

Source: Quora
It's also important to note that living organisms have evolved different strategies to optimize energy efficiency and minimize energy loss. For example, some organisms are able to enter a state of hibernation or dormancy to conserve energy during times of scarce resources. Others have developed mechanisms to recycle or reutilize energy-rich molecules, reducing the need for constant energy input.
Energy & Metabolism
As always, energy is neither created nor destroyed. Most energy is “lost” in the form of heat due to the use of the energy for a variety of metabolic processes. This will come into play when food webs are discussed in Unit 8 - Ecology. ⚡
In biological systems, energy is essential for the maintenance of life. Energy-related pathways in these systems are designed to allow for a controlled and efficient transfer of energy throughout the organism. These pathways are generally sequential in nature, with the products of one reaction serving as the reactants for the next step in the pathway.
One of the key principles of energy metabolism is that energy is stored in molecules, and that this energy can be converted from one form to another through a series of chemical reactions. These reactions are organized into metabolic pathways, which are series of enzyme-catalyzed reactions that take place in a specific order to convert energy from one form to another.
One example of such a pathway is the process of cellular respiration, which is the process by which cells convert glucose and oxygen into carbon dioxide, water and adenosine triphosphate (ATP). This process is composed of several sequential steps, each catalyzed by specific enzymes. The process starts with the breakdown of glucose, which is converted into pyruvate through a series of reactions. This process is called glycolysis. 🍬
The pyruvate then enters the citric acid cycle, where it is converted into acetyl-CoA, and then into CO2 and H2O, releasing energy in the form of ATP. The final step of cellular respiration is the electron transport chain, where the energy released in the citric acid cycle is used to produce ATP through the process of oxidative phosphorylation.
Another example of a metabolic pathway is the process of photosynthesis, where plants convert light energy into chemical energy in the form of glucose. This process occurs in two stages: the light-dependent reactions and the light-independent reactions. In the light-dependent reactions, light energy is converted into chemical energy in the form of ATP and NADPH. These products are then used in the light-independent reactions, also known as the Calvin cycle, to convert CO2 into glucose.
These examples illustrate that energy-related pathways in biological systems are sequential and tightly regulated to allow for a more controlled and efficient transfer of energy. 🌿
By breaking down large and complex molecules into smaller ones in a step-by-step manner, and using the energy released in each step to drive the next, organisms can maximize the amount of energy they can extract from their food. Additionally, by using the products of one reaction as the reactants for the next, organisms can conserve resources and minimize the waste of energy.
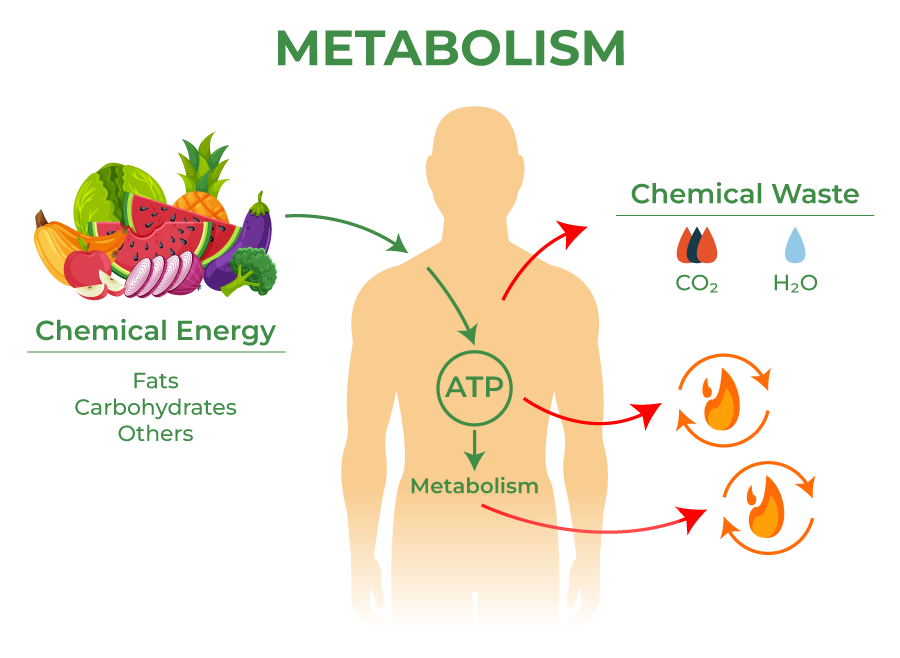
Source: Geeks for Geeks
Likewise, energy is gained through eating. All organisms get their energy from plants, regardless of whether or not the organism consumes plants. Carnivores still obtain their energy from plants, it has just been transferred from a plant to the organism that they are eating. 🥩
Check out the AP Bio Unit 3 Replays or watch the 2021 Unit 3 Cram
Browse Study Guides By Unit
🧪Unit 1 – Chemistry of Life
🧬Unit 2 – Cell Structure & Function
🔋Unit 3 – Cellular Energetics
🦠Unit 4 – Cell Communication & Cell Cycle
👪Unit 5 – Heredity
👻Unit 6 – Gene Expression & Regulation
🦍Unit 7 – Natural Selection
🌲Unit 8 – Ecology
📚Study Tools
🧐Exam Skills

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.