What do I need to know about the Periodic Table?
2 min read•july 11, 2024
AP Chemistry 🧪
269 resourcesSee Units
The periodic table of elements can be your best friend in AP Chemistry if you understand its components.
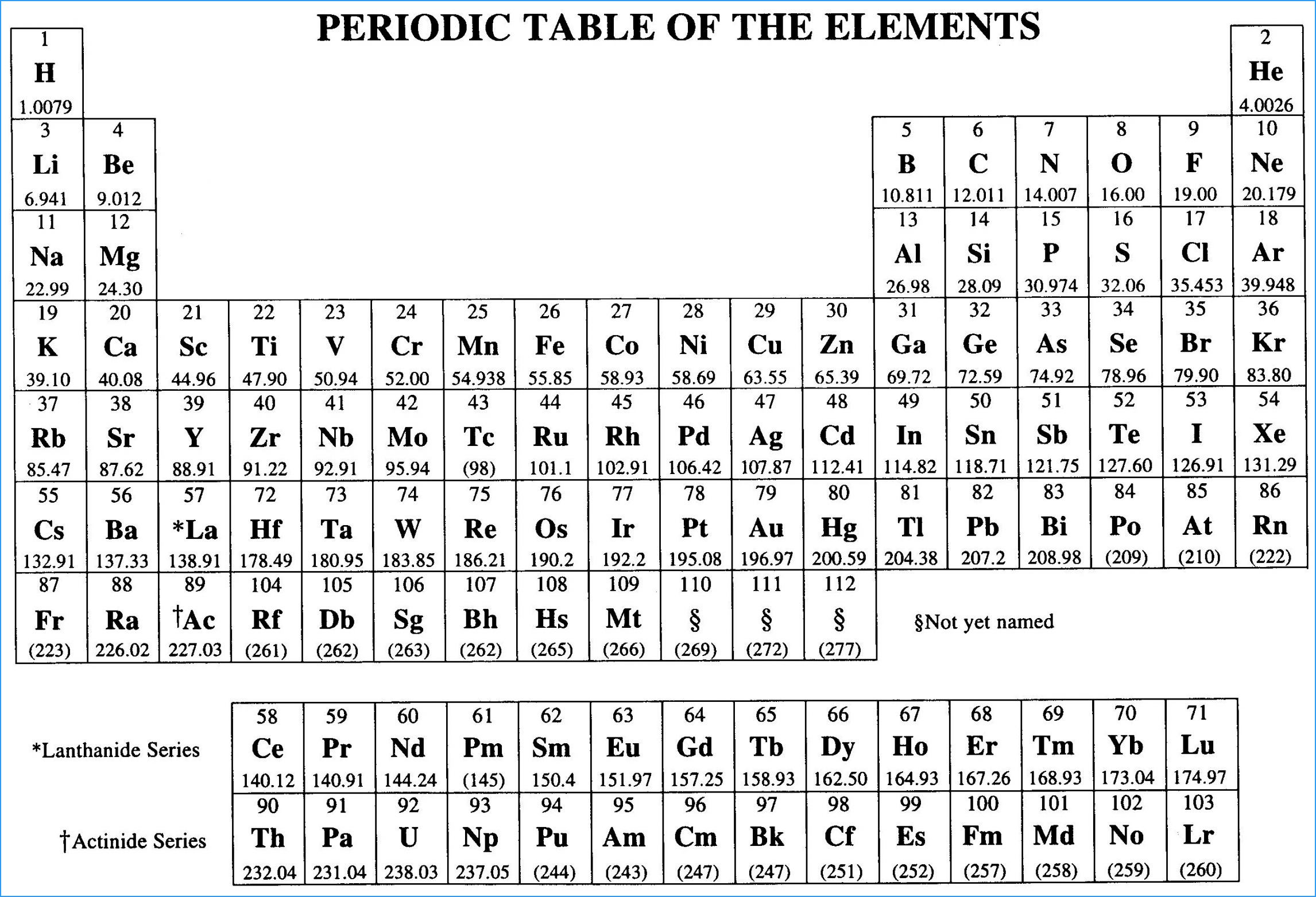
Squares
⭐️ Each square on the periodic table represents a different element.
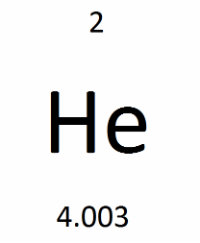
The 2 here represents the atomic number of the element, He {Helium} is the element's symbol, and 4.003 is the atomic mass of the element.
⭐️ ATOMIC NUMBER = a whole number that represents the number of protons in the element's nucleus
⭐️ ELEMENT SYMBOL = a commonly used representation of the element
⭐️ ATOMIC MASS = the element's mass (protons + neutrons), which is not a whole number because it is a weighted average of the masses of the naturally occurring isotopes of the element
Metals, Nonmetals, and Metalloids
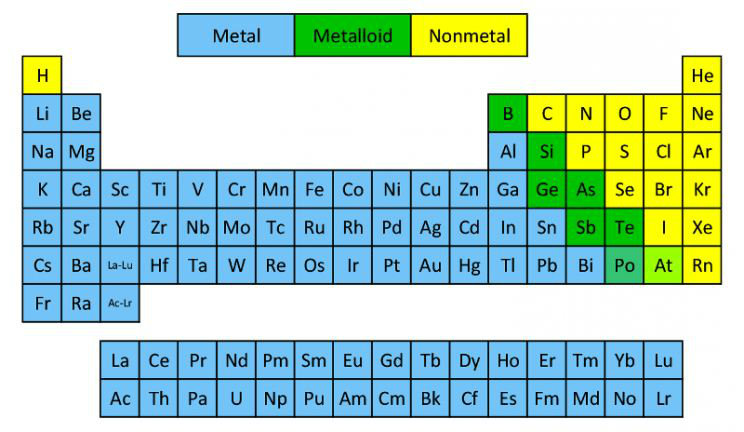
⭐️ METALS, shown in blue, are usually solids and are good conductors of heat and electricity. Chemically speaking, metals tend to lose electrons in reactions.
⭐️ METALLOIDS, shown in green, have properties of metals AND nonmetals. They are useful in the computer industry.
⭐️ NONMETALS, as shown in yellow, often have the opposite properties of metals. They are poor conductors of heat and electricity, and tend to gain electrons in chemical reactions.
⭐️ As you can see, there is a staircase of metalloids which divides the metals from the nonmetals (excluding Hydrogen). On the AP Chemistry exam, your periodic table will not be colored indicating what element is what, so it is important to be able to readily identify these properties.
Periods and Groups
⭐️ PERIODS are the horizontal rows on the periodic table, and the elements in each period have consecutive atomic numbers. The periods are numbered from one to seven.
⭐️ GROUPS are the vertical rows on the periodic table. The elements in each group will have similar chemical properties due to their shared number of valence electrons (electrons in the outermost shell of the atom). On some periodic tables, there are roman numerals at the top of each group to indicate this number of valence electrons.
Want to learn more?
Make sure to check out this Overview of AP Chem Unit 1: Atomic Structure and Properties and for more details about the Periodic Table, review the 🎥 important periodic trends of chemistry video replay.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.