What is Formal Charge?
2 min read•july 11, 2024
AP Chemistry 🧪
269 resourcesSee Units
An Atom's Formal Charge
Formal charge is the charge assigned to an atom in a molecule.
- Sometimes, compounds can have multiple structures.
- Generally, the structure that minimizes formal charge is the best structure to represent the actual molecule because it costs the lowest energy.
- That being said, you don't have to worry about formal charge on the exam unless a question specifically asks you about you.
How is it calculated?
- Formal charge is equal to (valence electrons) - (lone pair electrons + bonds)
Example 1: CO₂
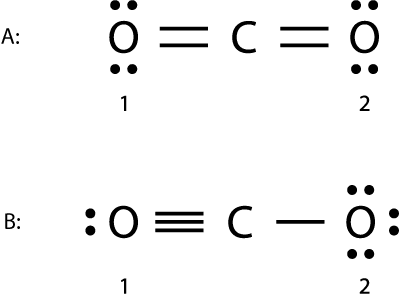
Structure A
- Formal charge for O1: (6 valence electrons) - (4 lone pair electrons + 2 bonds) = 0
- Formal charge for C: (4 valence electrons) - (0 lone pair electrons + 4 bonds) = 0
- Formal charge for O2: (6 valence electrons) - (4 lone pair electrons + 2 bonds) = 0
Structure B
- Formal charge for O1: (6 valence electrons) - (2 lone pair electrons + 3 bonds) = +1
- Formal charge for C: (4 valence electrons) - (0 lone pair electrons + 4 bonds) = 0
- Formal charge for O2: (6 valence electrons) - (6 lone pair electrons + 1 bonds) = -1
Structure A is a better representation of CO₂ because it minimizes formal charge.
Example 2: Fulminic Acid
- From question 2 on the 2017 free response:
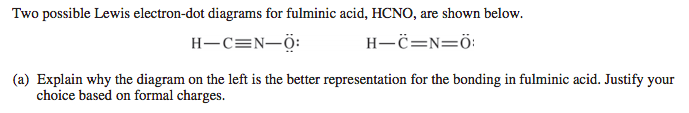
- And here are the scoring guidelines:

- You'll notice that both structures have an atom with a formal charge of -1.
- When this happens, the structure with the charge on the more electronegative atom is the better representation.
Want more practice? Make sure to check out the tips and tricks for AP Chem FRQs and this video replay about 🎥 Resonance and Formal Charges.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.