Dalia Savy
AP Chemistry 🧪
269 resourcesSee Units
Now that we've got precipitation reactions down, let's move on to the next primary type of chemical reactions! The second main type of reaction that you learn in this unit is the acid-base neutralization reaction. 🍊
Defining Acids & Bases
There are many different ways to define the behavior of acids and bases. The AP Chemistry curriculum focuses on the Brønsted-Lowry definition. When you think "Brønsted-Lowry," you should immediately focus on the transfer of a proton or hydrogen ion.
A Proton
First things first, how is a proton equivalent to a hydrogen ion? 🤔
A proton is a subatomic particle that is found in the nucleus of an atom. It has a positive electric charge and is one of the fundamental building blocks of matter.
Remember, an ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electric charge. A hydrogen ion is a hydrogen atom that has been stripped of its electron, resulting in a net positive charge: H+. Because a proton is identical to a hydrogen ion, the terms "proton" and "hydrogen ion" are often used interchangeably in chemistry.
Sometimes, you may even see H₃O⁺ in place of H⁺.
Brønsted-Lowry Definitions
Focusing on the transfer of a proton, acids are proton donors while bases are proton acceptors. What you basically see happen is a hydrogen ion being transferred from a substance, denoted as the acid, to another substance, denoted as the base.
Since acid-base reactions are just transfers of hydrogen ions, who says they can't go both ways? They usually can, although they do often shift in a certain direction. This begins to cover content that is discussed later in this course, so let's keep it simple!
If acid-base reactions can go back and forth, there must be an acid and a base on both the reactant and product side. This creates conjugate acid-base pairs. Looking at a chemical equation, you should be able to tell what the acid-base pairs are and pick out the conjugates.
Let's focus on the following example: H₂O + H₂S → H₃O⁺ + HS⁻.
First things first, what are the acid-base pairs?
First pair: H₂O and H₃O⁺
Second pair: H₂S and HS⁻
Now, which are the acids, and which are the bases? A quick way to know would be to figure out which compound in the pair has an additional hydrogen. Since H₃O⁺ has one more hydrogen than H₂O, it is the conjugate acid. This makes H₂O the base.
Try the second pair on your own! 😊
👉 If you'd like more practice with this concept and to learn more about the Brønsted-Lowry definitions, make sure to review our study guide about titrations.
Amphiprotic Substances
There are also these weird agents called amphiprotic substances. They can both donate and accept protons! An example that you really, really know is H₂O, but NH₃⁻ is also an amphiprotic substance.
The reason why they are amphiprotic is that they have both a lone pair that can accept and bond with a proton and a transferable proton that they can donate as well.
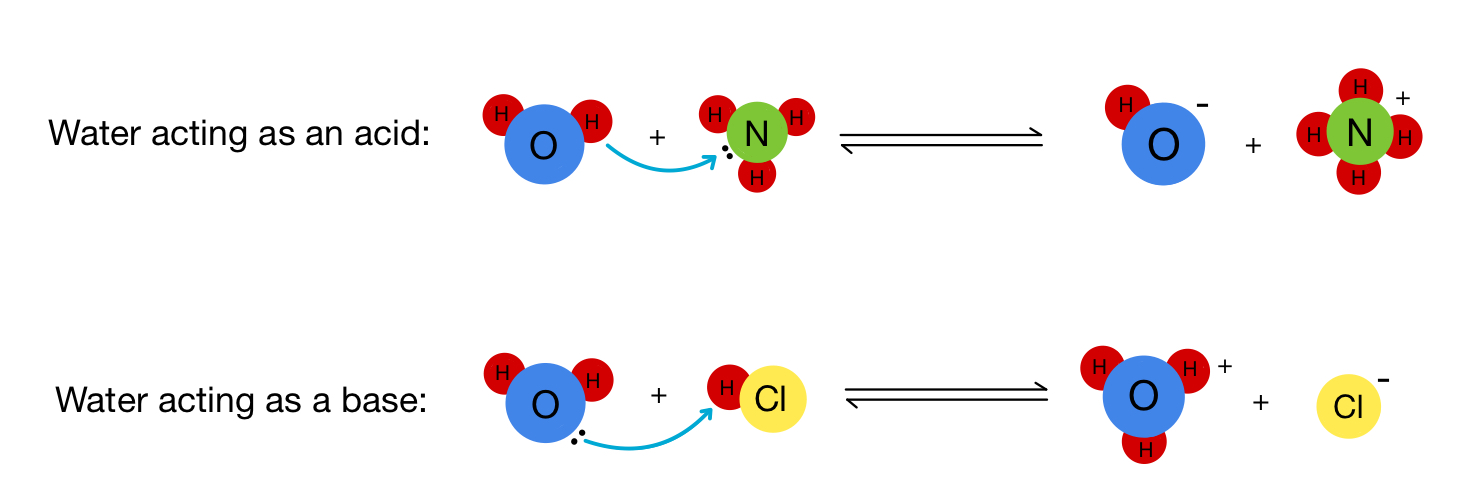
Image Courtesy of Expii
Acid-Base Neutralization
A neutralization reaction occurs when an acid and base react to often form an ionic salt and liquid water. The H⁺ ion from the acid combines with the OH⁻ from the base to form H₂O (l). The basic form of the reaction is acid + base → salt + water.
You usually have to write out the chemical reaction. Let's say the two reactants are HNO₃ (aq) and KOH (aq), what are the products? 💭
To make things easier on yourself, automatically write out H₂O (l) since you know that a proton is transferred to form water. Then, just combine the remaining ions, which would form the salt: HNO₃ (aq) + KOH (aq) → H₂O (l) + KNO₃ (?)
Soluble Salt?
Using solubility rules, is KNO₃ soluble? Or is it a precipitate? Any compound with NO₃ is soluble, so KNO₃ is in the aqueous state: HNO₃ (aq) + KOH (aq) → H₂O (l) + KNO₃ (aq)
Net Ionic Equation
We're back to net ionic equations! Remember, a net ionic equation is a chemical equation that shows only the species precipitating in a chemical reaction, omitting the spectator ions. For review on this subject, be sure to check out an earlier study guide in this unit that focuses primarily on net ionic equations.
⚠️ So far, we've been practicing writing net ionic equations for precipitation reactions. In doing so, we only dissociated soluble salts. Here, in neutralization reactions, we have to be really careful not to dissociate weak acids and bases. This is because they only partially dissociate into their constituent ions. Make sure you remember the strong acids and bases!
| Strong Acids | Strong Bases |
| HCl | CaOH |
| HBr | SrOH |
| HI | BaOH |
| HNO₃ | Group 1 metal + OH⁻ |
| H₂SO₄ | |
| HClO₃ | |
| HClO₄ |
Luckily, HNO₃ is a strong acid and KOH is a strong base, so we can dissociate both completely in the chemical equation. This makes the complete ionic equation the following: H⁺ (aq) + NO₃⁻ (aq) + K⁺ (aq) + OH⁻ (aq) → H₂O (l) + K⁺ (aq) + NO₃⁻ (aq)
We're so close to done! Now, all you have to do is eliminate the spectator ions (K⁺ and NO₃⁻), and you get the corresponding net ionic equation: H⁺ (aq) + OH⁻ (aq) → H₂O (l)
Concentration of Ions Question
With an acid-base neutralization reaction, you could also find the concentration of the ions. With this chemical reaction, you specifically focus on the concentrations of the hydrogen ion and hydroxide ion. In other words, what is [H⁺]? [OH⁻]?
Let's say we are given the following information and are expected to solve for the concentration of the hydrogen and hydroxide ions: 0.250 M and 28.0 mL of HNO₃ and 0.320 M and 53.0 mL of KOH.
Mole Calculations
Let's find the number of moles of HNO₃ and KOH using the equation for molarity.
Molarity = moles / volume in L - We have to convert the volumes we have into L by dividing by 1000.
HNO₃: 0.250 M = x moles / 0.0280 L
x = 0.00700 moles of HNO₃
KOH: 0.320 M = x moles / 0.0530 L
x = 0.0170 moles of KOH
Limiting Reactant
The limiting reactant is the reactant that there is less of. In this case, since there is a one-to-one ratio for all compounds, we can quickly identify HNO₃ as the LR.
Ion with a Concentration of Zero?
Since H⁺ is in both the limiting reactant and H₂O, it has a concentration of 0. The spectator ions cannot have a concentration of 0. Remember, they just help the reaction take place.
Half the question is done🥳! [H⁺] = 0.
[OH⁻]?
Finding the concentration of the excess compound is often the hardest part of the problem. First, we have to find the number of moles that reacted by converting the LR into the product. Again, since everything is one-to-one, we don't have to do extra stoichiometry. The number of moles that reacted is 0.00700.
Then, we simply subtract from the number of excess moles we started with, which is 0.0170 moles of KOH.
0.0170 - 0.00700 = 0.010 moles unreacted.
Last but not least, we need a volume! 28.0mL + 53.00mL = 0.081 L
0.010 moles unreacted / 0.081 L = 0.12 M of OH⁻
Final Answers
[H⁺] = 0
[OH⁻] = 0.12
Practice, practice, practice! It's just a lottttt of stoichiometry🙃.
Net Ionic Equation Practice
Write the net ionic equation of a reaction between HNO₃ and Al(OH)₃.
Here are the steps you should take:
- Write out the products: H2O + Al(NO3)₃
- Balance the equation: 3HNO₃ + Al(OH)₃ → 3H2O + Al(NO₃)₃
- Write out the states of matter using solubility rules: 3HNO₃ (aq) + Al(OH)₃ (s) → 3H2O (l) + Al(NO₃)₃ (aq)
- Al(OH)₃ is insoluble! We cannot dissociate it in the next step
- Dissociate aqueous substances: 3H⁺ (aq) + 3NO₃ (aq) + Al(OH)₃ (s) → 3H₂O (l) + Al⁺³ (aq) + 3NO₃⁻ (aq)
- Identify spectator ions: 3H⁺ (aq) + 3NO₃ (aq) + Al(OH)₃ (s) → 3H₂O (l) + Al⁺³ (aq) + 3NO₃⁻ (aq)
- Cross out spectator ions: 3H⁺ (aq) + Al(OH)₃ (s) → 3H₂O (l) + Al⁺³ (aq)
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.