K
Krish Gupta
Daniella Garcia-Loos
AP Physics 2 🧲
61 resourcesSee Units
Density 🧊
Solids have a definite shape and a definite volume. However, the same is not true for all fluids. Hence, we need a different quantity when dealing with fluid mechanics: density. Density is the ratio of mass per volume of a certain object. The most commonly used units for density are kilograms per meter cubed ($kg/m^3$). In physics, we denote density with the greek letter p (rho).
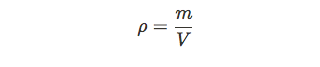
One important distinction to be made here is that mass and volume are extensive properties, which means the amount of substance will change the value. But density is an intensive property, meaning if you have a balloon full of helium or a 100 tanks full of helium, the density of helium will remain the same.
Density is a measure of the mass of an object per unit of volume. Here are some key points about density:
- Density is defined as the mass of an object divided by its volume. It is usually expressed in units of grams per cubic centimeter (g/cm^3).
- Density is an intrinsic property of an object, meaning that it does not depend on the size or shape of the object.
- Density is affected by the mass and volume of an object. An object with a higher mass or a smaller volume will have a higher density.
- Density can be used to compare the relative densities of different materials. For example, gold is denser than aluminum, meaning that it has a higher mass per unit of volume.
- The density of a substance can be calculated using the formula density = mass/volume.
Example Problem #1:
A block of metal has a mass of 50 grams and a volume of 10 cubic centimeters. What is the density of the metal?
To solve this problem, you will need to use the density formula to calculate the density of the metal.
Density = mass/volume = 50 g/10 cm^3 = 5 g/cm^3
Example Problem #2:
A cylinder of wood has a mass of 200 grams and a radius of 2 centimeters. What is the density of the wood?
To solve this problem, you will need to use the density formula and the formula for the volume of a cylinder to calculate the density of the wood.
Volume of cylinder = πr^2h = 3.14 x (2 cm)^2 x h = 4πh
Density = mass/volume = 200 g/(4πh)
Example Problem #3:
A swimming pool has a volume of 50,000 liters and a mass of 400,000 grams. What is the density of the water in the pool?
To solve this problem, you will need to use the density formula to calculate the density of the water in the pool.
Density = mass/volume = 400,000 g/50,000 L = 8 g/L
Example Problem #4:
Predict the density of ice relative to water at 0 degrees Celsius, and design an investigation to verify your prediction.
Prediction: The density of ice will be less than the density of water at 0 degrees Celsius.
Explanation: At 0 degrees Celsius, water is a solid (ice) and a liquid. The molecules of a solid are more closely packed together than the molecules of a liquid, so the density of a solid is typically higher than the density of a liquid. However, the molecules of ice are arranged in a lattice structure that has a more open, less dense arrangement than the molecules of water. As a result, the density of ice is less than the density of water at 0 degrees Celsius.
Investigation: To verify this prediction, you could conduct the following investigation:
- Obtain a container of water and a container of ice.
- Measure the mass and volume of the water and ice using a balance and a graduated cylinder or other appropriate measuring tools.
- Calculate the density of the water and ice using the formula density = mass/volume.
- Compare the densities of the water and ice.
- If the density of the ice is less than the density of the water, your prediction is supported. If the density of the ice is greater than or equal to the density of the water, your prediction is not supported.
Commonly Asked Questions About Density
Which has greater density: 100 grams of mercury of 10000 kilograms of mercury?
They same have the same density. The density is a constant associated with a type of substance.
Density is the property that determines if something will float or sink in a given liquid. If the density of the object is greater than the density of the liquid, then it will sink. If the density of the object is less than the density of the liquid, then it will float. 🤯
If object A and object B have the same density , but we have more volume of object B than object A, do we have more mass of object A or B?
If the densities are the same than the ratios of mass and volume should be the same. So since B has more volume it must have more mass!
The technical term for the ratio of densities (of the object of interest to a liquid or some reference object) is called the specific gravity. It is important to realize that specific gravity is a property of interaction and not of just one object. For example, it is not possible to find the specific gravity of wood. However, you can find the specific gravity of wood relative to water or relative to oil. Focus on the word relative. 🤔
So to summarize, if the specific gravity of an object is more than 1 in water then it will sink in water. Similarly, if the specific gravity of an object is less than 1, it will float. Specific gravity will also tell us what fraction of the object is submerged and how much is above water.
Q. A sponge has a specific gravity of 0.1 in water. What percent of the sponge is above the water’s surface when placed in water. 🧽
A. 90%. Since the sponge has a specific gravity of less than 1 it will float in the water. The specific gravity is 0.1 which means about 10% of the sponge will be submerged in the water. Hence, 90% of the sponge (and it’s volume) will be above the surface of the water. 💧
Browse Study Guides By Unit
💧Unit 1 – Fluids
🔥Unit 2 – Thermodynamics
⚡️Unit 3 – Electric Force, Field, & Potential
💡Unit 4 – Electric Circuits
🧲Unit 5 – Magnetism & Electromagnetic Induction
🔍Unit 6 – Geometric & Physical Optics
⚛️Unit 7 – Quantum, Atomic, & Nuclear Physics
📆Big Reviews: Finals & Exam Prep
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.