1.5 Structure and Function of Biological Macromolecules
6 min read•november 18, 2024
Jed Quiaoit
Danna Esther Gelfand
AP Biology 🧬
358 resourcesSee Units
Four Types of Macromolecules: A Refresher
There are four main types of macromolecules: nucleic acids, carbohydrates, lipids, and proteins. These macromolecules are the building blocks of cells and perform a wide range of functions in living organisms.
Nucleic acids are made up of nitrogenous bases, sugars, and phosphate groups, and they carry genetic information. There are two types of nucleic acids: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). DNA stores genetic information, while RNA carries out the instructions of DNA and helps to synthesize proteins. 🧬
Carbohydrates are composed of carbon, hydrogen, and oxygen atoms, and they serve as a source of energy and structural support in cells. Examples of carbohydrates include sugars, starches, and cellulose. 🍩
Proteins are large, complex molecules made up of amino acids, and they perform a wide range of functions in cells, including catalyzing chemical reactions, transporting molecules, and providing structural support. Proteins can be found in all cells and tissues, and they play a key role in many processes within the body. 🥩
Lipids are composed of carbon, hydrogen, and oxygen atoms, and they are important for energy storage and cell membrane structure. Examples of lipids include fats, oils, and phospholipids. 😁
Structure Determines Function
The subunits, or monomers, that make up a polymer play a crucial role in determining the structure and function of the macromolecule. The directionality of the monomers, or the way in which they are arranged and bonded together, can affect the overall shape and conformation of the polymer.
For example, in a protein, the primary structure refers to the specific sequence of amino acids that make up the protein, and this sequence determines the three-dimensional structure of the protein, or its tertiary structure. The tertiary structure, in turn, determines the protein's function. Similarly, the arrangement of monomers in a carbohydrate or a nucleic acid can affect the structure and function of the polymer.
🧬 Nucleic Acids
Nucleic acids, such as DNA and RNA, are long, linear polymers made up of nucleotide monomers. The nucleotides in DNA and RNA are linked together through covalent bonds between the sugar and phosphate groups of adjacent nucleotides. The sequence of nucleotides in a nucleic acid molecule is important because it carries the genetic information that is used to build and maintain living organisms.
The sequence is read in a specific direction, starting at the 5' end and ending at the 3' end. This directionality is important because it determines the sequence of nucleotides that will be synthesized during DNA replication and RNA transcription.
During these processes, nucleotides are added to the 3' end of the growing strand by a process called polymerization, which involves the formation of a covalent bond between the 3' hydroxyl group of one nucleotide and the 5' phosphate group of the next nucleotide.
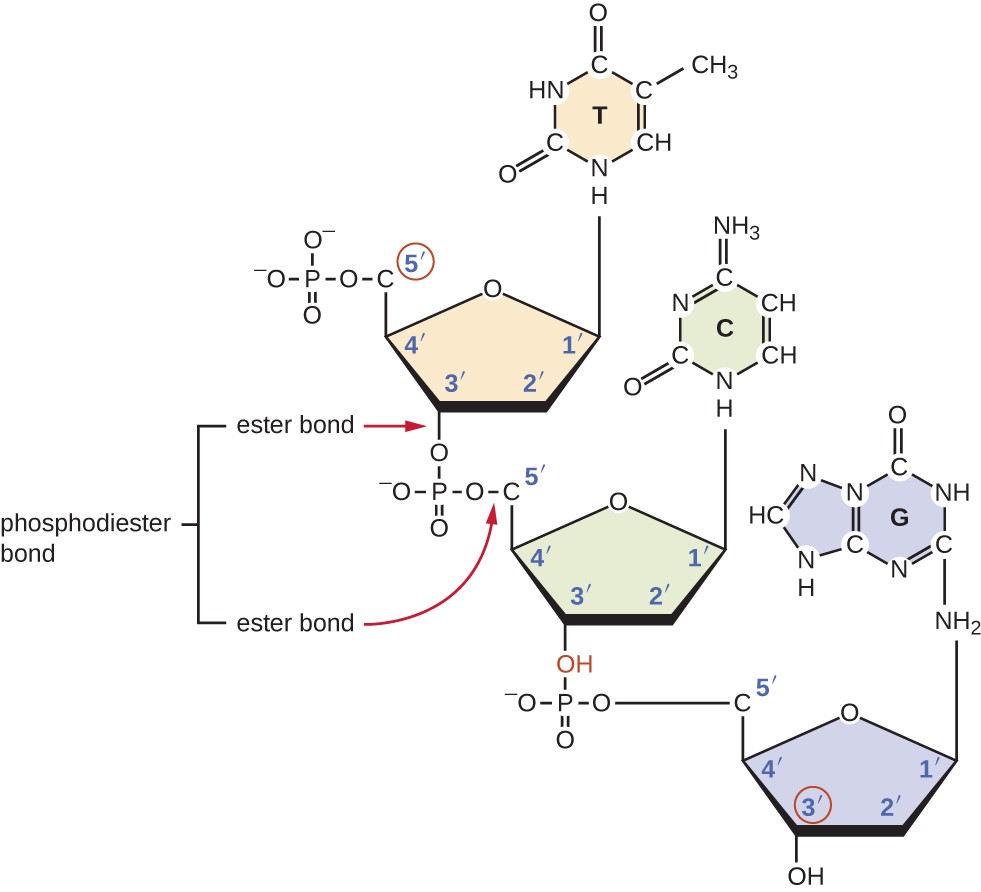
Image courtesy of Lumen Learning
Likewise, DNA is a double-stranded helical molecule that is composed of two antiparallel strands of nucleotides that are held together by hydrogen bonds between the bases.
The DNA molecule is shaped like a twisted ladder, with the sugar-phosphate backbone forming the sides of the ladder and the bases forming the rungs. The strands are oriented in opposite directions, with one strand running from the 5' end to the 3' end and the other strand running from the 3' end to the 5' end. This antiparallel orientation of the strands is important for the stability of the DNA molecule and for its role in genetic information storage and transfer.
The bases in DNA are adenine (A), cytosine (C), guanine (G), and thymine (T). These bases are held together by hydrogen bonds, with A pairing with T through two hydrogen bonds and C pairing with G through three hydrogen bonds. This base pairing is specific and complementary, and it ensures the stability of the DNA molecule and the accuracy of genetic information transfer.
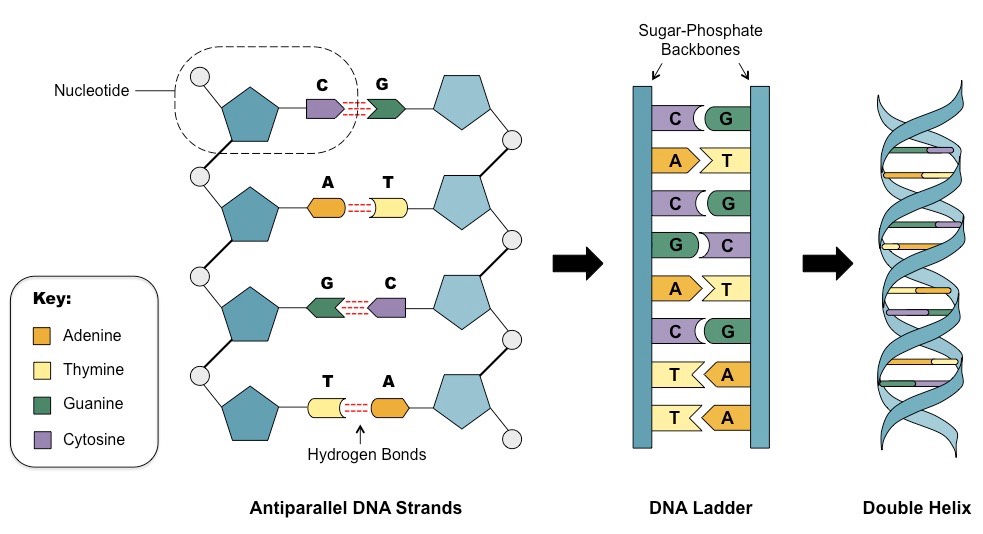
Image courtesy of BioNinja
🥩 Proteins
Proteins are linear polymers made up of amino acids that are linked together by peptide bonds. A peptide bond is a covalent bond that is formed between the carboxyl group of one amino acid and the amino group of another amino acid. The formation of a peptide bond results in the release of a molecule of water, and this process is called peptide bond formation or peptide bond synthesis.
The sequence of amino acids in a protein is called the primary structure of the protein, and it is determined by the sequence of the nucleotides in the gene that encodes the protein. The primary structure of a protein is important because it determines the three-dimensional structure of the protein, or its tertiary structure, which in turn determines the protein's function.
Varied functions of proteins include, but are not limited to: structural, catalytic, signaling, defense, and transport within cells. Functioning as: enzymes, hormones, storage, transport (through membranes), defense proteins, and receptor proteins.
Levels of Protein Structure
- Primary Structure – a sequence of amino acids, peptide bonds.
- Secondary Structure– the result of hydrogen bonding between the components of the polypeptide backbone, the carboxyl and amino functional groups along the peptide chain-forming alpha helix or beta-pleated sheet
- Tertiary Structure – the result of interactions between the alpha-helix and Beta pleated sheet (interactions of the same polypeptide chain) (van der Waals forces, hydrophobic interactions, hydrogen bonding, disulfide bridges, etc. cause its formation).
- Quaternary Structure – interactions between two or more polypeptide chains forming the multi-subunit protein. (common examples you will come across: DNA polymerase, hemoglobin)
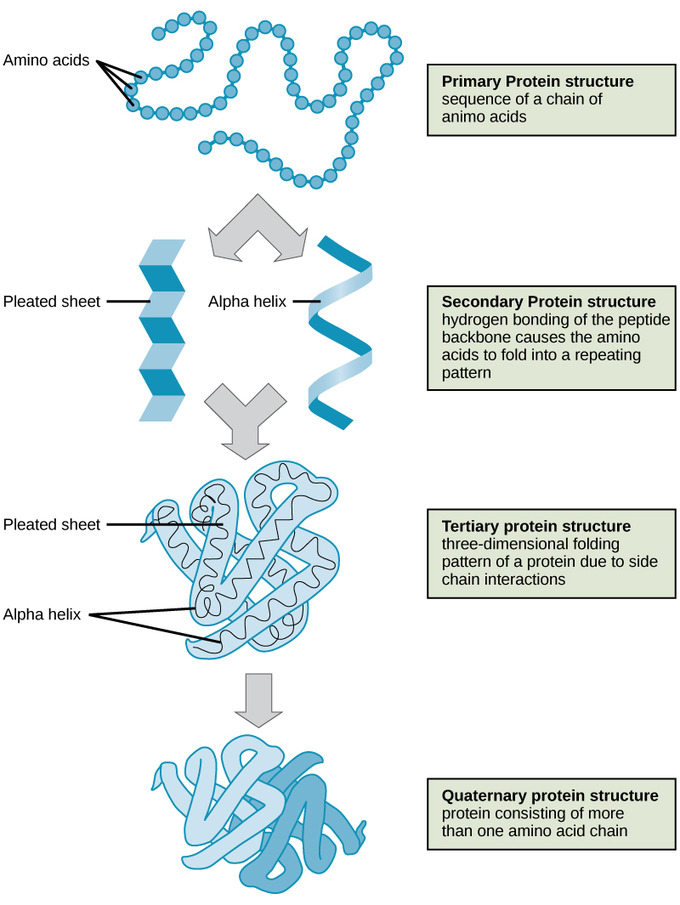
Image courtesy of Lumen Learning.
Denaturation
Denaturation is a process that occurs when proteins lose their tertiary structure, which is the three-dimensional arrangement of their amino acid residues, due to the disruption of the non-covalent interactions that hold the protein in its native conformation. This can be caused by various factors such as heat, pH changes, and the presence of certain chemicals or enzymes.
Denaturation can result in the loss of the protein's biological activity, which is why it is considered an inactive form of protein. Note that this process is different from hydrolysis, which is the breaking down of proteins into smaller peptides or amino acids by the action of water or enzymes.
💡 Important connections to other units: Sickle-cell disease, an inherited blood disorder is caused by a single amino acid substitution in the protein hemoglobin.
Species that share a common ancestor have some similar structured proteins and their amino acids correspond to each other.
🍩 Carbohydrates
Simple carbohydrates, also known as monosaccharides, contain a single sugar unit, while complex carbohydrates, also known as polysaccharides, contain multiple sugar units linked together.
Linear carbohydrates are those that have a straight chain of sugar units, while branched carbohydrates have branches coming off of the main chain. The structure of a carbohydrate can affect its physical and chemical properties, as well as its function in the body.
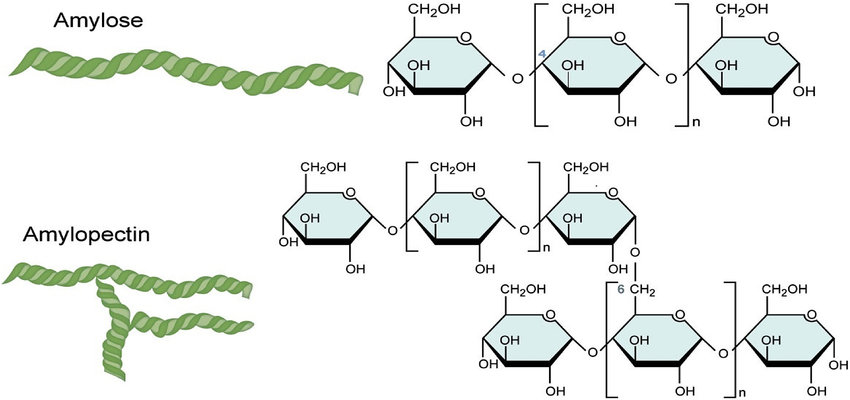
Image courtesy of ResearchGate
Carbohydrates play important roles in many biological processes, including energy metabolism, cell communication, and structure and function of cells and tissues. They are also an important source of energy for the body, providing fuel for the brain, muscles, and other organs.
Other Things of Notable Importance
More About Carbs: Disaccharides and Polysaccharides
Disaccharide – two monosaccharides joined together by a covalent bond, formed through dehydration synthesis (Di – two).
Most Common Disaccharides
- Maltose - disaccharide of two glucose monosaccharides combined together by dehydration synthesis.
- Sucrose - disaccharide of glucose monosaccharide and a fructose monosaccharide combined together by dehydration synthesis.
- Lactose - disaccharide of glucose monosaccharide and a galactose monosaccharide combined together by dehydration synthesis.
The covalent bond that the monosaccharides are joined together by is called a glycosidic bond, it forms both disaccharides and polysaccharides.
Polysaccharides are polymers of sugars that have functions of storage and structure which are determined by the positions of the glycosidic bonds and the monomers in the sugar polymers. (macromolecules that hold between 100-1000 monomers)
Most Common Types of Polysaccharides and Their Functions
- Starch – stores energy in plants
- Glycogen – stores energy in animals; usually stored in muscle and liver cells.
- Cellulose – structural polysaccharide; an important part of the cell wall in plants.
- Chitin – structural polysaccharide; found in exoskeletons of arthropods and cell walls of fungi.
Check out the AP Bio Unit 1 Replays or watch the 2021 Unit 1 Cram
Browse Study Guides By Unit
🧪Unit 1 – Chemistry of Life
🧬Unit 2 – Cell Structure & Function
🔋Unit 3 – Cellular Energetics
🦠Unit 4 – Cell Communication & Cell Cycle
👪Unit 5 – Heredity
👻Unit 6 – Gene Expression & Regulation
🦍Unit 7 – Natural Selection
🌲Unit 8 – Ecology
📚Study Tools
🧐Exam Skills

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.