2.2 Intramolecular Force and Potential Energy
6 min read•june 18, 2024
Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
You may be thinking, what are intramolecular forces? Intramolecular forces are the forces between two atoms in a molecule! This is very different from intermolecular forces, which we learn in unit three.
💡 Intramolecular and intermolecular forces are often confused, so here are some tips:
- Intermolecular forces are those between molecules. Think inter = between two groups
- Intramolecular forces are those between two groups in a molecule, so think intra = within a group.
So far, we learned about two intramolecular forces: covalent bonds and ionic bonds.
Potential Energy and Bonding
You may wonder where potential energy fits into bonding. Well, remember how chemistry favors and always strives to reach the highest stability? The lower the potential energy of a bond, the more stable it is! This is a fundamental rule you should try to keep in the back of your mind when learning about the strength and formation of bonds.
Because of this connection, physical or chemical processes can be described through energy diagrams. A graph of potential energy versus the distance between atoms is a useful tool for understanding the interactions between atoms. Taking a look at this graph, you can see several things:
- The "equilibrium bond length" - basically another phrase for the distance between atoms where potential energy is at its lowest point. Let's simplify it even more: the distance at which the atoms are the most stable.
- The bond energy - the amount of energy necessary to separate two atoms in a bond. This can be calculated or conceptually thought of as the difference in potential energy between the separated atoms and the atoms at their equilibrium bond length, or most stable phase.
- The strength of the bond - this can be grasped from the bond energy. In general, bonds with higher bond energies are stronger and more stable, while bonds with lower bond energies are weaker and less stable.
- The length of the bond, or the physical distance between the two atoms bonded to one another.
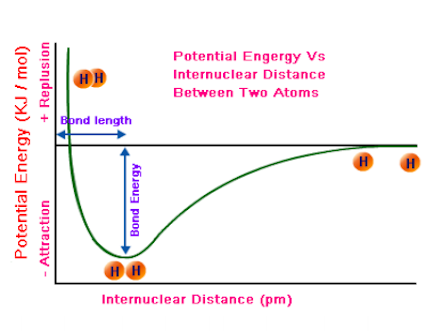
Image Courtesy of Stack Exchange
Potential Energy and Covalent Bonds
In molecular compounds with covalent bonds, the bond length is influenced by both the size of the atoms and the bond order.
Bond order is another term for how bonds can either be classified as single, double, or triple bonds.
| Bond Order | Electrons Involved in Bond | Bond Length | Bond Energy |
| Single Bond (-) | Two electrons | Longest | Smallest |
| Double Bond (=) | Four electrons | Middle | Middle |
| Triple Bond (≡) | Six electrons | Shortest | Largest |
An easy way to remember the number of electrons involved in a bond is that each dash on a lewis dot diagram corresponds to two shared electrons.
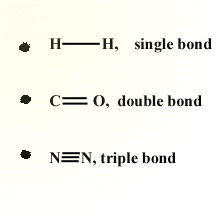
Image Courtesy of Shodor
Since bonds with higher bond energies are stronger and more stable, triple bonds are generally the most stable. This makes them the most difficult to break. However, it is important to note that stability also depends on other factors such as the size and charge of the atoms involved.
Let's try and put some of this information together and take a look at a graph of potential energy.
👀 Breaking Down a Potential Energy Diagram
For covalent bonds, the bond length is influenced by the bond order (single, double, triple) and the balance between repulsive and attractive forces. Bond energy in the diagram shows how the greatest potential energy is the repulsion of two atoms.
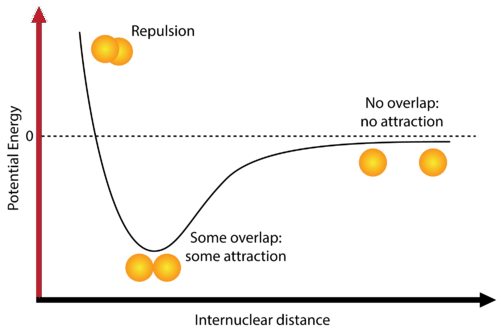
Image Courtesy of SplainScience
Let's take a look at each of these stages:
- Repulsion - Since the atoms are very close together and the internuclear distance is very small, the atoms are experiencing lots of electron-electron repulsion. This causes the bond to be very unstable and leads to a potential energy of greater than zero.
- Some overlap/attraction - This is the most stable state and the bond length at this point is what we referred to as the "equilibrium bond length." There is a balance⚖️ between the repulsive and attractive forces and a stable bond is formed. Hopefully, now you understand why potential energy is lowest when the bond is stable.
- The potential energy at this stage is the amount required to break the bond or the bond energy.
- No overlap/attraction - Since the internuclear distance is so large, there are no interactions between the two atoms, and no bond is formed. This leads to a potential energy of almost zero.
Example with PE Diagrams
It is good to understand these properties because you may be asked to guess where an element falls on this graph.
Say the following image is a diagram of chlorine atoms bonded together (Cl-Cl). Where would Br-Br fall in comparison to chlorine's curve?
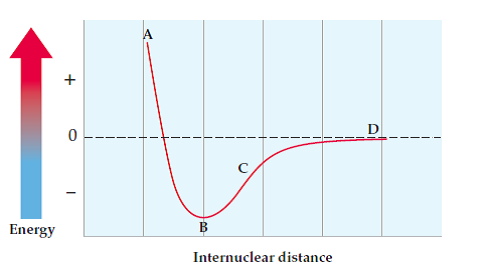
Image Courtesy of Chegg
To answer this question, we have to think about periodic trends and take a look at the axes of this graph:
- Internuclear distance: is the Cl-Cl bond or Br-Br bond longer? Well, the bond between the atoms with larger atomic radii would have to be the longer one. As you go down in a group on the periodic table, the atomic radius increases. Since bromine is below chlorine on the periodic table, the Br-Br bond is longer than the Cl-Cl bond.
- This tells you where to draw the Br-Br curve in relation to the x-axis.
- Potential energy: which bond would be easier to break? Cl-Cl or Br-Br? This should automatically make you think of ionization energy. The lower the ionization energy, the easier it would be to break the bond. As you go down a group, ionization energy decreases because there are more occupied electron shells and the nucleus' attraction with the valence electrons is weakest. Therefore, bromine has a lower ionization energy and the Br-Br bond is much easier to break.
- This tells you where to draw the Br-Br curve in relation to the y-axis.
Knowing that the Br-Br bond is longer and easier to break, you would have to graph its curve up (less energy) and to the right more (larger internuclear distance).
This question is a very good way to test your knowledge about this key topic and periodic trends. Here is what the graph should look like:
Forces Within Ionic Bonds
Understanding the strength of ionic interactions involves the use of Coulomb's Law.
As we went over in the previous unit, you do not need to know the formula. However, you are expected to understand what Coulomb's Law conceptually means.
Essentially, the energy of two interacting charged particles (ions) depends on the magnitude of charge and the distance between the nuclei of the two particles.
- The greater the charge of the atoms, the stronger the attraction. This is because the more positively charged a nucleus is, the more strongly it can attract electrons toward it.
- The closer the two particles are to each other, the stronger the attraction. Think about it this way: magnets are not attracted to each other if they are placed 3 feet apart. They need to be close to feel the attraction. 🧲
Smaller and highly charged ions have the strongest interactions, according to Coulomb's Law.
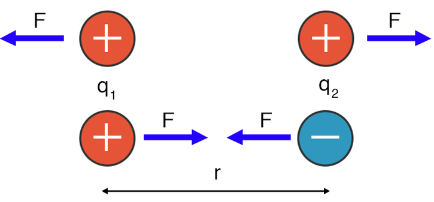
Image Courtesy of Science Facts
Take a look at this diagram. Don't worry too much about the variables, but F represents the force, q1 and q2 represent the magnitude of charges of the respective atoms, and r represents the distance between the nuclei of the atoms.
This image is basically showing you that Coulomb's law is the foundation behind "opposites attract." Coulomb's Law is everywhere!
Attraction occurs if the charges are opposite (positive and negative), while repulsion occurs if the charges are the same (positive and positive or negative and negative).
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.