Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Now that you've learned about the structure of an atom and the properties of electrons, we have to discuss how to draw molecules! By doing this, we can observe how the structure of an atom impacts the way it bonds.
What are Lewis Diagrams?
Lewis diagrams, or Lewis structures, are a way of drawing molecular structures and showing the present valence electrons and bonds. Lewis structures serve as one of the most important topics in this unit and the course as a whole, with the ability to draw out any molecule opening the door to thousands of other possibilities.
Lewis diagrams are used to predict the shape of a molecule and the types of chemical reactions it can undergo. They are based on the octet rule, which states that atoms tend to form bonds in such a way that they have a full valence shell of eight electrons.
Lewis diagrams are a type of localized electron model, meaning the electrons do not move freely throughout the structure. In a Lewis structure, the valence electrons are divided into two categories:
- Lone pairs: pairs of electrons that are localized around a single atom and are not shared with any other atoms.
- Bonding pairs: pairs of electrons found in the shared space between atoms (often represented by a dash)

Drawing Lewis Dot Structures (LDS)
Ionic LDSs
Ionic Lewis dot structures are very easy to draw out since ionic bonds form due to a transfer of electrons!🥳
- First, write the empirical formula of the compound down to see which elements are involved and how many atoms of each.
- Then, draw the metals and nonmetals with their respective electrons (you could do this mentally too once you get a hang of this process).
- Transfer valence electrons to the nonmetal (could be done mentally as well).
- Draw brackets around the lewis dot structures of the cation and anion and draw the charges outside of the brackets.
Example: NaBr
Sodium Bromide
- Going through the steps, sodium bromide's formula is NaBr. This tells you that there is only one atom of each element present in the LDS.
- You would remember that Na has 1 valence electron and Br has 7 valence electrons by looking at the groups they are in on the periodic table.
- When an ionic bond forms, 1 valence electron from Na is transferred to Br to create a full octet on both atoms, now ions. You have now created a sodium cation and a bromide anion, so you must show the charges on each outside the brackets.
Here is the LDS:
Image Courtesy of Wayne Breslyn
Try drawing the lewis dot structure of magnesium chloride. We'll give you the answer at the end!
Covalent LDSs
Covalent bonds are a little more difficult to draw out because the electrons are shared.
👉Here are some steps to guide you:
- Look at the empirical formula and count the number of valence electrons there should be total.
- Draw the central atom (in most cases it is carbon or the atom that is not hydrogen)
- Draw the outside atoms and put single bonds connecting atoms together.
- Draw full octets on each atom. Most atoms have 8 electrons when most stable.
- Count the valence electrons present so far
- If there are too many electrons, erase lone pairs from adjacent atoms and make double/triple bonds
- If there are too few electrons, add some to the central atom (see exceptions section⭐)
Here are a few examples, but we'll go through some more using these steps!
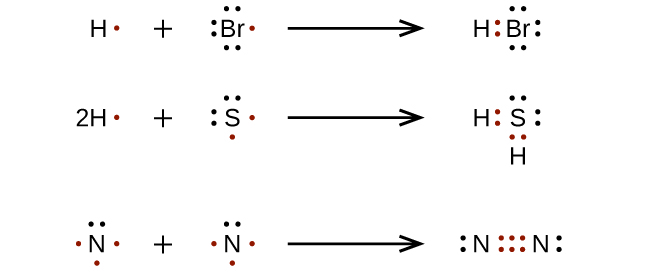
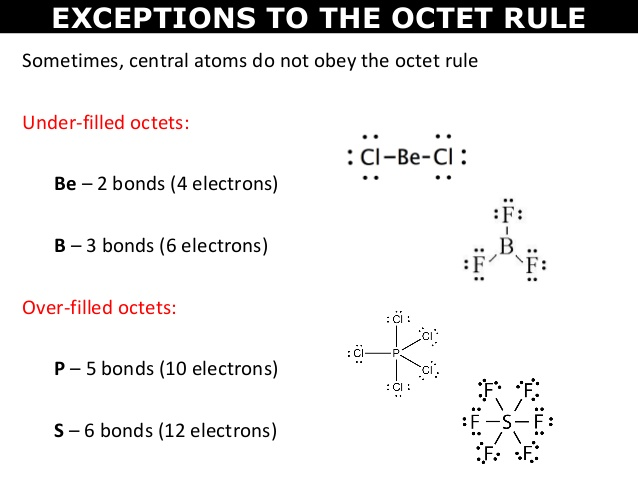
⭐Exceptions to the Octet Rule
There CAN be exceptions to the rules, so be careful when drawing Lewis dot structures.
- Some atoms have fewer electrons than a full octet of 8. Hydrogen can have a maximum of two valence electrons, beryllium can have a maximum of four valence electrons, and boron can have a maximum of six valence electrons.
- If there are too few electrons in your drawing, you may break the octet rule on the central atom ONLY IF the element has an atomic number of 14 or greater (you would be able to add extra electrons to the central atom to meet the required number of valence electrons).
- Some atoms have an odd number of valence electrons, so they would not be able to neatly fit into the octet rule. A complete pairing of an octet would not be able to happen.
- Some compounds have multiple bonds between the atoms if there aren't enough electrons. For example, the compound CO2 is represented as a carbon atom joined to two oxygen atoms by double bonds.
Here are some examples of the first two bullets:
Examples of Lewis Structures
Let's go over some relatively straightforward compounds first!
1) Draw a Lewis dot structure for O2.
Here is what you should be thinking as you get used to drawing these:
- Looking at the periodic table, we can notice that oxygen is in group 16. This means it has six valence electrons and since there are two oxygen atoms, there should be 12 valence electrons in this diagram in total.
- Since there are only two oxygen atoms, we could just draw them side by side (there is technically no central atom here).
- Connect the two oxygen atoms with a single dash, which represents two valence electrons.
- Draw 3 lone pairs on both of the oxygen atoms so that they both have a full octet. Here is what you should have so far:
- Count the number of valence electrons in the diagram above. There are 14 of them right now, but we only want 12. Since there are too many electrons, we can convert this single bond into a double bond by erasing lone pairs from each atom.
Now to check our work, we can count the number of valence electrons. Since there are 12 total and the octet rule is fulfilled on both atoms, this is the proper lewis dot structure of O2.
Try drawing the lewis dot structure of N2. The answer will be provided at the end.
2) Draw a Lewis diagram for CS2.
- Looking at the periodic table, we know that C has 4 v.e. and S has 6 v.e.. This accounts for a total of 16 valence electrons since the carbon atom has four and each of the two sulfur atoms have six.
- Draw carbon, the central atom.
- Draw two sulfur atoms, connecting them to the carbon atom with a single bond (4 electrons so far out of 16).
- Draw full octets on all three atoms. Here's what it should look like so far:
- In this current diagram, there are a total of 20 valence electrons, but we need 16. Therefore, we should form two double bonds. Here is what the final LDS looks like:
3) Draw the Lewis diagram for XeF2.
- Xe has 8 v.e. and F has 7 each. Therefore, there is a total of 22 valence electrons in this compound.
- Xe is the central atom since there is only one atom of xenon.
- Draw two fluorine atoms on either side and connect them to xenon with a single bond. Draw 3 full octets again. Here's what it looks like so far:
- There is a total of 20 electrons; we need two more! This is where breaking the octet rule might need to happen. Since Xe has an atomic number of 54, which is much greater than 14, we can break the octet rule and add the necessary number of electrons to Xe. Here is what the final LDS looks like:
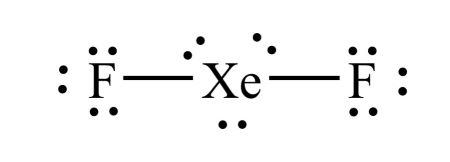
When you break the octet rule and have three lone pairs and two bonds, make sure that your lone pairs stay together. For example, you cannot have three valence electrons on one side of the xenon atom and three on the other side. They must remain in pairs of two. The following diagram is incorrect:
Try drawing the lewis dot structure of the polyatomic ion NH4+.
Answers to Practice Questions
1) Draw the LDS for Magnesium chloride
You always want to draw out the empirical formula first and make sure the charges cancel out to be 0 because magnesium chloride actually has 2 Cl atoms! Mg has a +2 charge while Cl has a -1 charge, so the compound is MgCl2. Here is the lewis dot structure:
Image Courtesy of Wayne Breslyn
You could also draw only one Cl atom, with a 2 coefficient outside of the brackets (indicating there are two chlorine ions). Don't confuse the term "coefficient" with "subscript" or "superscript."
2) Draw the LDS for N2.
Once you go through all the steps, you'll notice that there are 14 valence electrons. We only need 10 though since each nitrogen atom has five valence electrons, so we have to form double or triple bonds. If you draw a double bond, you'd still notice that we don't have 14 valence electrons, so there should be a triple bond.
Image Courtesy of Clutch Prep
3) Draw the LDS for the polyatomic ion NH4+.
Since the compound has a charge, we would just have to take one electron away. A positive charge indicates an absence of electrons, while a negative charge indicates an addition of electrons.
Here, it looks like there would be 9 valence electrons but since there is a +1 charge, there should only be 8 valence electrons total.
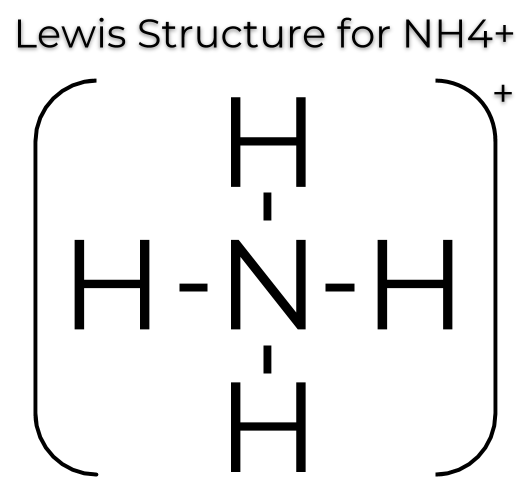
Image Courtesy of MaketheBrainhappy
These lewis dot structures get slightly more complex in the next key topic, but practice makes perfect! Try to master these examples before moving forward. 😀
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.