Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
This unit is all about molecular and ionic compound structure and properties! Now that we've discussed the types of bonds, intramolecular forces, and the structure of ionic solids, it is time to discuss metals and alloys! 🪙
Metallic Bonding
Although ionic and covalent substances are more common, you should be familiar with metallic substances as well and their lattice of cations surrounded by a ‘sea’ of valence electrons. 🌊
Sea of Electrons
When metals ionize, they lose a valence electron and become positive ions or cations. Metallic bonding can therefore be represented as an array of cations surrounded by this "sea" of valence electrons.
What this means is the nucleus and core electrons of the metal stay in place, but the valence electrons are very mobile, allowing them to be thought of as a sea.
Electrons usually belong to a certain atom but in metals, they move so much that they don't belong to one, single atom.
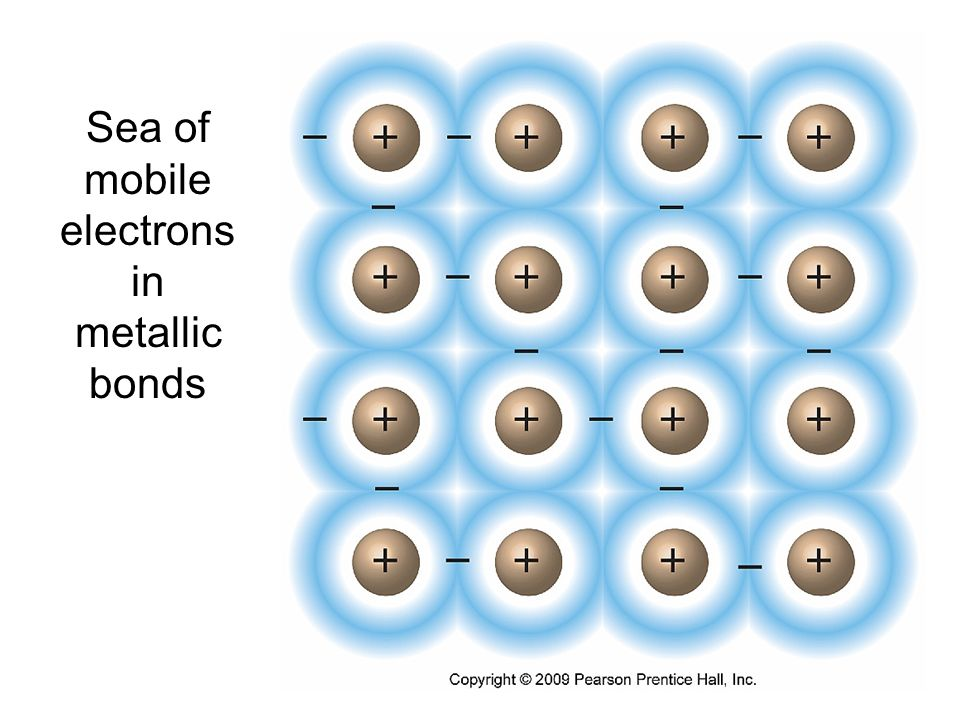
Since the valence electrons are free to move throughout the entire metallic structure, metallic substances have very unique properties:
- ⚡ Good conductors of electricity - The valence electrons in metals are delocalized. This simply means that they are mobile and can move freely throughout the entire structure. This allows metals to carry an electric current and conduct charge or, as we'll see in a future unit, be used in redox reactions.
- 🌡️ High melting and boiling points - These metallic bonds are very strong and require a lot of energy to be broken. Imagine boiling a metal such as gold or iron🤔
- 🌟 Shiny appearance - The way light reflects off delocalized electrons in a metal causes many metals to appear shiny.
- 🔌Malleability and ductility - Malleability reflects the metal's ability to be deformed and shaped without breaking. Ductility reflects a metal's ability to be bent and spun into a wire. These characteristics occur because the structure of metals is much less rigid than that of ionic solids. They can more easily be rearranged.
Comparing Solids
When comparing properties among the different solids, remember this chart:
| Type of Solid | Form of Unit Particles | Forces Between Particles | Properties | Examples |
| Molecular🧊 | Atoms or Molecules | LDFs, dipole-dipole, hydrogen bonds | fairly soft, low melting point, bad conductor | Argon, methane, sucrose, dry ice |
| Covalent-Network💎 | Atoms connected in a network of covalent bonds | Covalent bonds | Very hard, very high melting point, bad conductor | diamond, quartz |
| Ionic🧂 | Positive and negative ions | Electrostatic attractions | Hard and brittle, high melting point, bad conductor | salts (NaCl) |
| Metallic✨ | Atoms | Metallic bonds | varying hardness and melting points, good conductor, malleable, ductile | metals! Cu, Fe, Al |
Table Courtesy of unknown source
Right now, you should only be very familiar with the two bolded rows. The others are covered in unit three in more depth when we discuss intermolecular forces!
Alloys
Metals can also bond with other elements and create alloys. Alloys can be formed when two or more elements, where at least one is a metal, are in their liquid form being mixed together. When this mixture cools, the alloy is created. In order to produce a certain alloy, pure metals and elements must be mixed in a specific ratio. Each combination of substances produces an alloy with very unique characteristics.
There are two types of alloys you should be familiar with.
🍳 Interstitial Alloys
Interstitial alloys form when smaller atoms fill the interstitial spaces between larger atoms. The best example is steel, a substance that you probably haven't realized is an alloy! ⚙️
Steel is made up of iron and carbon atoms. Iron is a common base element for interstitial alloys, and carbon is a common alloying element. These properties reflect their atomic radii. In steel, carbon fits into the interstices of iron.
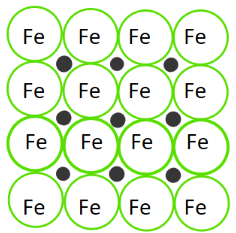
Image Courtesy of Study; The little black dots represent carbon atoms.
The properties of a sample of steel all depend on the ratio of carbon to iron. The amount of carbon that one uses to make steel can vary, allowing one to make different samples of steel.
Interstitial alloys, like steel, are known for their strength and hardness. This is because of the small size of the interstitial atoms (like carbon) that give the resulting alloy a high density. Think about it this way: you're stuffing small pieces into an array of atoms. This only makes the substance more dense and stronger!
🎺 Substitutional Alloys
Substitutional alloys form when an atom of one element substitutes an atom of another element of similar size. This is different from interstitial because there are no additional atoms being added to the array, atoms are rather replaced.
The most well-known example of a substitutional alloy is brass. Copper is a common base element for substitutional alloys, and zinc is a common alloying element. In brass, zinc is substituted for copper. 🎷
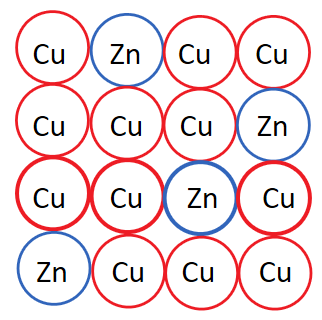
Image Courtesy of Study
Substitutional alloys, like brass, are known for their good electrical and thermal conductivity. These two properties result from the presence of delocalized electrons in the crystal lattice.
Interstitial vs. Substitutional Alloys
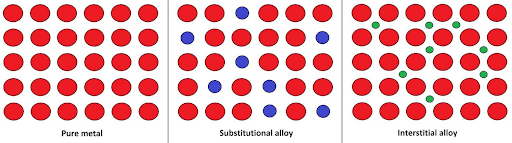
Image Courtesy of Chemistry LibreTexts
The biggest difference is that with an interstitial alloy, you are adding smaller atoms, but with a substitutional alloy, you are replacing atoms with ones that are similar in size.
Alloys are generally harder and stronger than pure metals because the added elements distort the structure and properties. Alloys are also less malleable than pure metals.
Check your Understanding
The following practice question is based on one posted by the Advanced Placement Youtube channel, and it goes over content reviewed in this guide and the previous guide.
(1) A student ran an experiment to see if the following solids conduct electricity.
| Solids | Does it conduct electricity? |
| Fe (s) | yes |
| FeCl2 (s) | no |
(a) Explain the results the student saw.
(b) Is there anything that could have been different in this experiment to see the FeCl2 sample conduct electricity?
Practice Question Sample Responses
The following are sample responses for part a:
- This student found that a sample of iron conducted electricity since it is a metal. Metals have delocalized valence electrons, usually displayed by the sea of electrons diagram, allowing them to be good conductors of electricity.
- The student found that the sample of FeCl2 didn't conduct electricity because it is an ionic solid. Ionic solids have a lattice structure. Therefore, electrons cannot move freely and the sample didn't conduct electricity.
The following refers to part b:
Recall that as long as there are mobile valence electrons, the sample will conduct electricity. There are two ways to ensure mobile valence electrons are present. Either of the following responses is acceptable:
- Melt the FeCl2 solid and then test it for conductivity. The liquid FeCl2 would conduct electricity since the ions would be mobile and able to flow.
- Dissolve the FeCl2 solid in water. In an aqueous solution, the ions are able to flow and conduct electricity.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.