Atoms are the most basic unit of matter. When several atoms interact with each other, they can form molecules. Depending on how the electrons of these atoms interact, a covalent bond or an ionic bond could form.
Everything in chemistry strives to become the most stable possible. Atoms do the same thing! Atoms bond in order to reach a more stable, lower energy state. ⚖️
Valence electrons are typically involved in bonding,
not core electrons! They play a crucial role in the bonding process and determine the chemical properties of the resulting molecule. Here is a quick review of everything we learned about valence electrons in
unit one:
Valence electrons are the outermost electrons in an atom.
Valence electrons are found in the s and p orbital of the outermost occupied electron shell.
A gap in
ionization energies could tell us how many valence electrons an element has.
Elements in the same group on the periodic table have the same number of valence electrons and therefore form similar molecules with other elements.
Image Courtesy; This table displays the successive ionization energies of elements in period three on the periodic table. Here you can see the jump in ionization energies and how that corresponds to the number of valence electrons an atom has.
As
we discussed in unit one, electronegativity is one of the five periodic trends you should be familiar with. Here is what you should know before learning about bonding:
Electronegativity values increase as you go from left to right across a period on the periodic table ➡️. This trend can be explained by the increasing atomic numbers across a period. The more protons there are in the nucleus of an atom, the stronger the nucleus' positive charge is. Nuclei with more protons are more effective at attracting electrons since opposite charges attract.
Electronegativity values decrease as you go down a group on the periodic table ⬇️. This trend is explained by the increasing atomic radius of the elements down a group. The larger the atom, the more distance between the nucleus and the electrons, weakening the attraction.
Coulomb's Law comes in handy when you want to measure the attraction between two atoms. It states that the strength of forces that hold atoms together depends on two factors:
Magnitude of charge - The greater the charge, the stronger the attraction.
Distance between the nuclei of the particles - The closer the two particles, the stronger the attraction.
This relates directly to the trend of electronegativity and basically explains it! As you go down a group, the distance between the valence electrons and the nucleus increases, which decreases the attractive force, and therefore, the atom's electronegativity. Think about it! 🙃
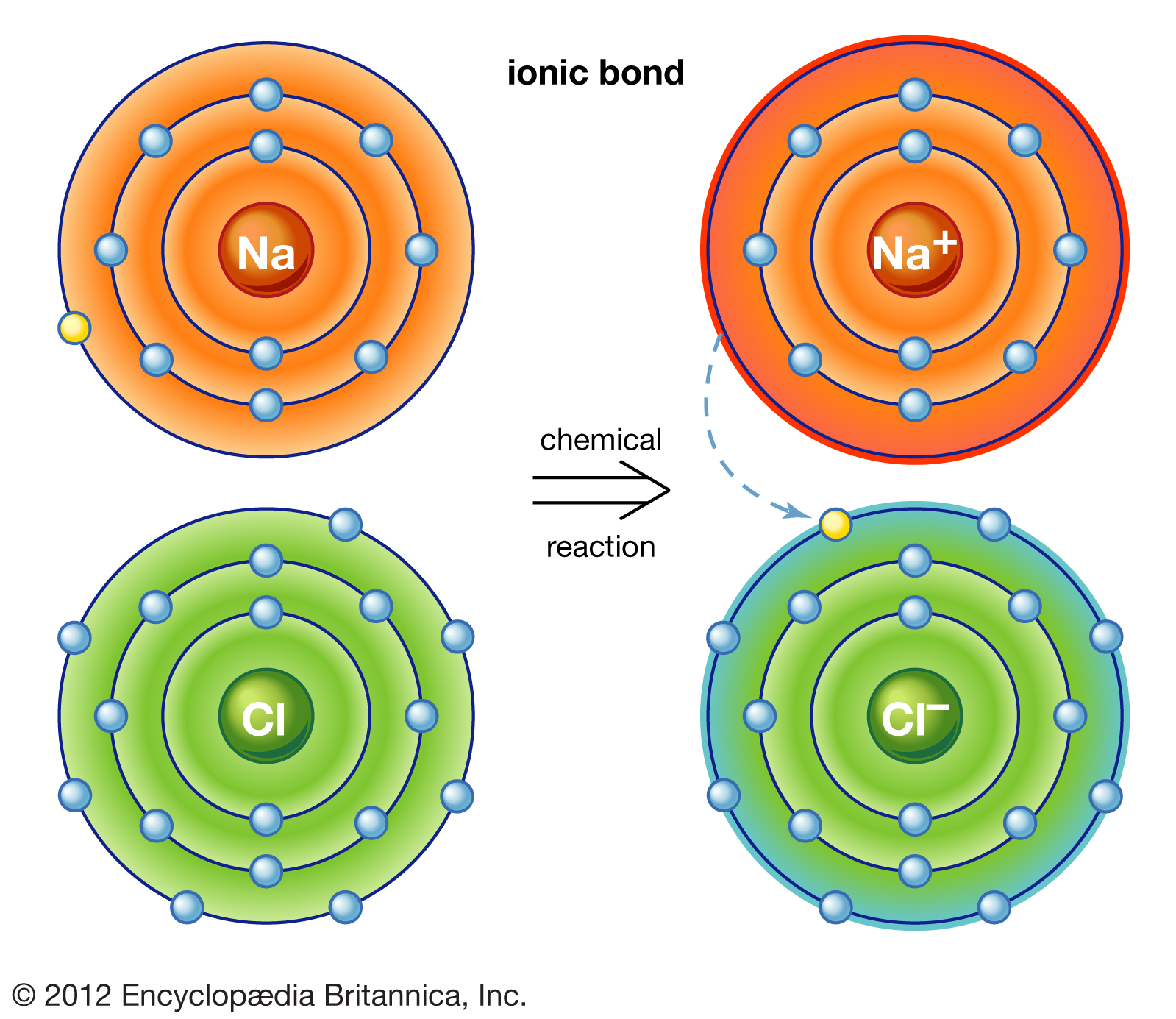
Ionic bonds are formed by the transfer of valence electrons from atom to atom, usually from a metal to a nonmetal. Let's take a look at an example of an ionic bond between a sodium and chloride atom! 🤓
Na(s) + ½ Cl2(g) → NaCl(s)
NaCl, a brittle salt with a high melting point was formed in this chemical reaction. Ionic bonds are held together not by shared electrons or a direct bond, but rather through electromagnetic forces that hold positive and negative ions together. These electromagnetic forces are so strong that it takes lots of energy to break them apart, hence the high boiling and melting points.
Ionic compounds also form a crystal lattice of ions, giving them their rigidity. In a crystal lattice, ions are arranged in a repeating, three-dimensional pattern. This crystal lattice is, again, held by strong electrostatic forces. This characteristic of ionic molecules makes them generally good electrical conductors. When an ionic compound is melted or dissolved in water, the ions can move freely within the crystal lattice structure, producing electricity! ⚡
Note that when sodium and chlorine interact to form an ionic bond, sodium gives a valence electron to chlorine. This gain and loss of an electron produces ions, hence the name "ionic bond." The atom that loses an electron, sodium, will gain a positive charge and is called a cation. The atom that gains an electron, chlorine, will gain a negative charge and is called an anion.
Remember that Coulomb's Law states that greater charges and smaller distances lead to the strongest attractions.
When asked which ionic compound would have a higher melting point, always look for differences in charge and size. The higher the charge of the ion, the stronger the negative-positive attraction is and the more energy it takes to break the bond. This increases both the melting and boiling point. Same goes for size!
TIP - Always look for differences in charge first; they have a greater impact on melting points.
Examples with Ionic Compounds and Melting Points
Which compound would have a higher melting point: MgF2 or NaF?
Looking at the charge first, you would notice that Mg has a +2 charge, while Na only has a +1 charge. This automatically means that MgF2 has a higher melting point than NaF.
Which ionic compound would have a higher melting point: LiF or NaBr?
Since both charges are the same (+1 / -1), the main difference would have to be the size of the ions. Keeping the
periodic trends in mind, lithium and fluorine are much smaller ions than sodium and bromine. Therefore, LiF must have a higher melting point.
Lithium and Fluorine are in period 2, while Na is in period 3 and Br is in period 4. Remember, when you go down on the periodic table, the atomic radii increase since there are more occupied electron shells. You may be asked to give a reason for this trend in free response questions🤔!
In covalent bonds, electrons are shared between two or more atoms (typically nonmetals). There are two different types of covalent bonds based on the electronegativities of the atoms involved:
If a polar covalent bond is formed, there is an unequal distribution of charge.
If a nonpolar covalent bond is formed, there is an equal distribution of charge. ⚖️️
We'll learn more about polarity when we get into
molecular geometry later in this unit.
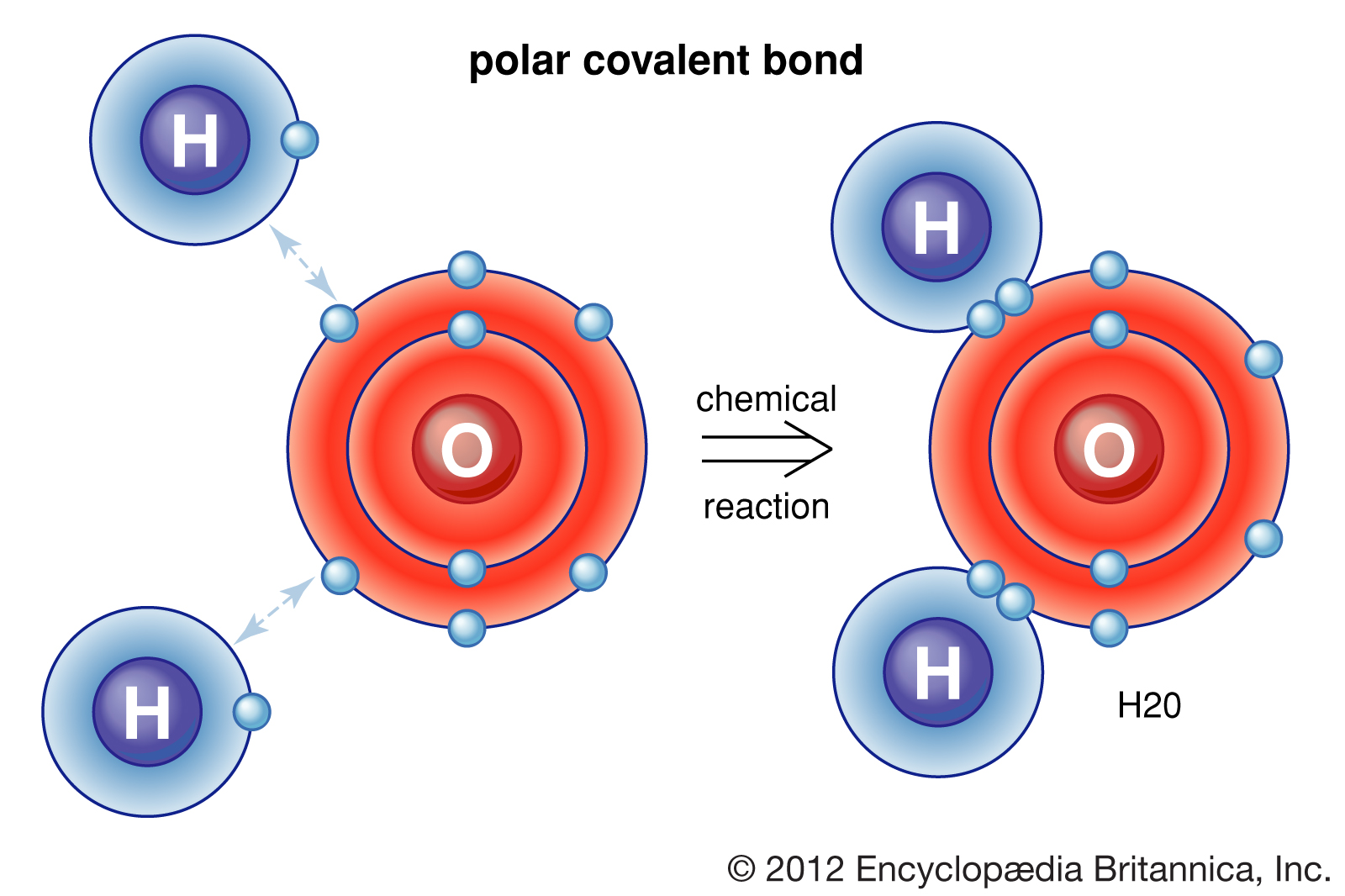
We could see two O-H polar covalent bonds here! These attractions are very strong and form water which, as you know, is essential for life on Earth.
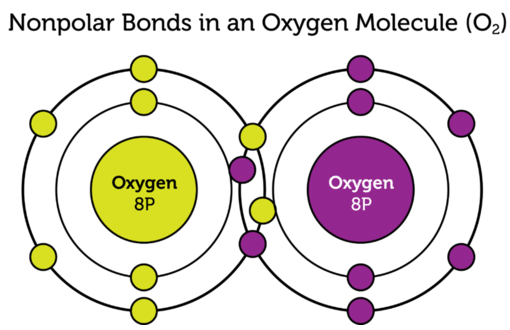
Image Courtesy of cK-12; In this oxygen molecule, there is a nonpolar covalent bond between the two oxygen atoms!
To analyze the difference between the two types of covalent bonds, we would need to look at electronegativity. Valence electrons shared between atoms of similar electronegativity constitute a nonpolar covalent bond. This is why the two oxygen atoms above form a nonpolar covalent bond. Since the electronegativity is the same, the oxygen nuclei pull on the other oxygen atom's electrons with the same strength. Think "nonpolar = balance!"
Valence electrons shared between atoms of unequal electronegativity constitute a polar covalent bond, like in a water molecule. Hydrogen has an electronegativity of 2.2 while oxygen has an electronegativity of 3.44. Therefore, oxygen attracts electrons more strongly and will pull the electrons towards it.
This unequal distribution of charge leads to oxygen developing a partial negative charge. This difference in electronegativity leads to bond dipoles, which are covered more in the next unit.
For now, just remember that greater differences in electronegativity within a bond lead to greater bond dipoles!
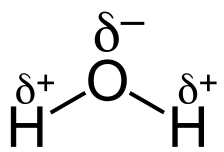
Image Courtesy of Socratic; δ, or lowercase delta, represents a partial positive or partial negative charge.
Here are some principles of ionic and covalent bonds that you should look out for when deciding what chemical bond will form:
Ionic Bonds
form between two elements that have an electronegativity difference of >1.7
usually form between a metal and a nonmetal
joins a cation (positive ion) and an anion (negative ion)
If a solid has a high melting point and is a good conductor of heat and electricity when dissolved in water, it is most likely an ionic compound. This is because of the concept of a free-flowing ion that generates electricity.
If a solid has a low melting point and doesn't conduct electricity in any state, it is most likely a molecular compound (which has covalent bonds).
There is one more circumstance: If a solid has a high melting point and doesn't conduct electricity in any state, it is a network solid made up of covalent bonds. Don't worry about this yet, it's covered
in future units :).