Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Valence Shell Electron Pair Repulsion (VSEPR)
Lewis structures can determine properties such as geometry, bond orders, bond lengths, and dipoles for molecules. The Valence-Shell-Electron-Pair-Repulsion (VSEPR) theory can predict molecular geometry by minimizing electron-electron repulsion. It specifically uses the Coulombic repulsion between electrons as a basis for predicting electron arrangement.
What do I need to know about VSEPR?
You should definitely memorize the table below for the AP Exam. It gives you everything you need to know about VSEPR and will answer a lot of questions that require memorization on the AP. Once you practice, the questions that involve the VSEPR Theory become free points🥳!
Let's go over what each column means:
- Family - think of family as how many groups of atoms or molecules branch off the middle atom (number of x + number of e in the general formula).
- General Formula - made up of three parts:
- M = middle atom
- X = attached atoms
- E = lone pairs
- Electron Domain Geography - gives you an idea of what the molecule looks like. It shows where the electrons or atoms are in relation to the middle atom, M.
- The graph below also includes angle measures that you should be aware of in that specific molecular geometry.
- Shape - This is the main column that you should memorize and learn to associate with the general formula, electron domain geography, and hybridization.
- Hybridization - You only have to memorize the hybridization of families 2, 3, and 4🧠.
VSEPR
Bonding
Sigma and Pi Bonds
Sigma (σ) bonds are covalent bonds where electrons are found shared on the internuclear axis. Hybrid orbitals form σ bonds, and they are stronger than π bonds.
Pi (π) bonds are covalent bonds where orbitals are perpendicular📐 to the internuclear axis. Unhybridized orbitals form π bonds.
You don't really have to know these definitions, but be aware of the following:
- A single bond is made up of 1 σ bond.
- A double bond is made up of 1 σ bond and 1 π bond.
- A triple bond is made up of 1 σ bond and 2 π bonds.
The more pi bonds in a molecule:
- The higher the bond energy
- The shorter the bond length
Examples
Count the number of σ bonds and the number of π bonds in the following two structures:
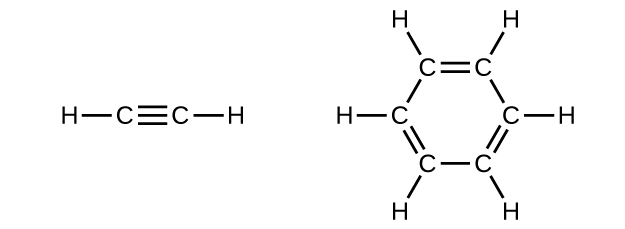
Image Courtesy of BC Open textbooks
In the molecule on the left, there is 1 triple bond and 2 single bonds. 1 triple bond is made up of 1 σ bond and 2 π bonds, while the single bond is only made up of 1 σ bond. Therefore, in total, there are 3 σ bonds and 2 π bonds in this molecule.
In the molecule on the right, there are 3 double bonds and 9 single bonds. This means this molecule is made up of 12 σ bonds and 3 π bonds.
Hybridization
Hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties. Hybridization is also an expansion of the valence bond theory💥.
There are 5 main hybridizations, 3 of which you'll be tested on: sp3, sp2, sp, sp3d, sp3d2. For these hybridizations, electron orbitals fuse together to fill subshells and go to a lower energy state. It also allowed for things like CH4, since technically the way the electron pairs are organized, 4 sigma bonds would not be possible.
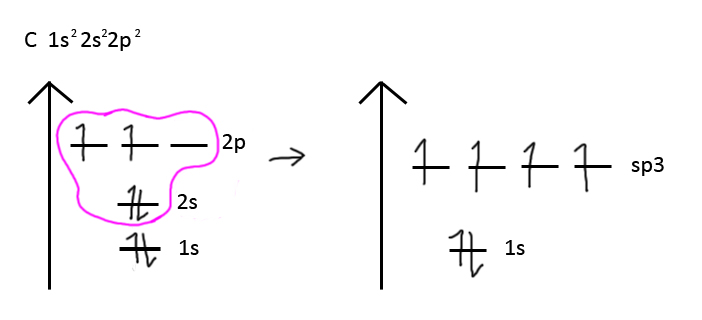
In the above example, carbon's 2p and 2s orbitals fuse into 4 half-filled sp3 orbitals that can make 4 sp3-orbital sigma bonds. The same principle applies to the other hybridizations.
AP Free-Response Questions
The following questions are from past AP Chemistry exams that were posted online by College Board.
2007 Examination - #6a-d
(a) In the box provided, draw a complete Lewis electron-dot diagram for the IF3 molecule.
(b) On the basis of the Lewis electron-dot diagram that you drew in part (a), predict the molecular geometry of the IF3 molecule.
(c) In the SO2 molecule, both of the bonds between sulfur and oxygen have the same length. Explain this observation, supporting your explanation by drawing in the box below a Lewis electron-dot diagram (or diagrams) for the SO2 molecule.
(d) On the basis of your Lewis electron-dot diagram(s) in part (c), identify the hybridization of the sulfur atom in the SO2 molecule.
2010 Examination - #5a-c
Use the information in the table below to respond to the statements and questions that follow. Your answers should be in terms of principles of molecular structure.
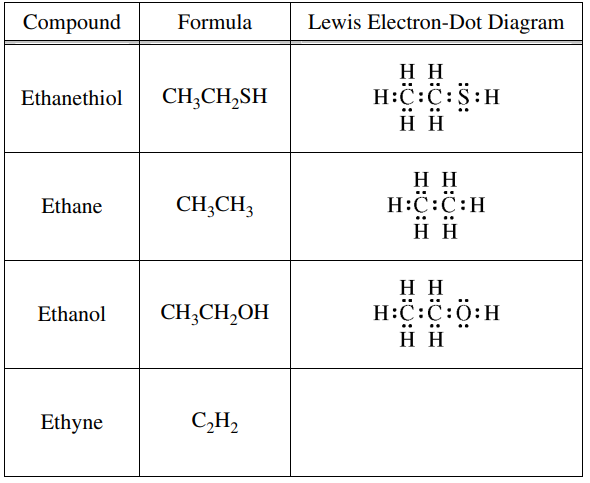
(a) Draw the complete Lewis electron-dot diagram for ethyne in the appropriate cell in the table above.
(b) Which of the four molecules contains the shortest carbon-to-carbon bond? Explain.
(c) A Lewis electron-dot diagram of a molecule of ethanoic acid is given below. The carbon atoms in the molecule are labeled x and y, respectively.
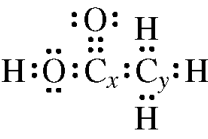
Identify the geometry of the arrangement of atoms bonded to each of the following.
(i) Carbon x
(ii) Carbon y
AP FRQ Scoring Guidelines
2007 Examination - #6a-d
For part a, one point is earned for a correct Lewis diagram, such as the one below. This example is done with solely dots, but you can also represent the bonds with lines.
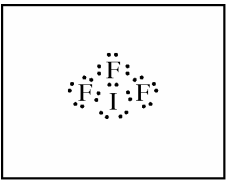
The correct answer for part b is "T-shaped." One point is earned for the correct molecular geometry of the diagram drawn in part a. If you drew the Lewis diagram incorrectly in part a, you may still earn credit in part b as long as your answer is consistent with your drawing.
Two points can be earned in part c. One point is earned with the correct Lewis structure and the other is earned for stating that both sulfur-oxygen bonds are double bonds.
One point is earned in part d for listing the correct hybridization: sp2. If you drew the Lewis structure incorrectly in part c, but your answer in part d was consistent with it, you would get credit in this part.
2010 Examination - #5a-c
One point is earned in part a for the correct Lewis structure drawn in the table.
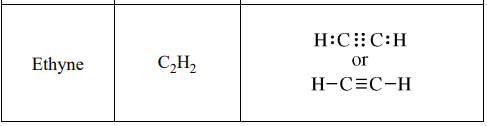
Two points can be earned in part b: one for the correct choice and one for the correct explanation. The following is a sample response that would earn both points: "Ethyne, which contains a triple bond, has the shortest C-to-C bond. The other molecules have single C-to-C bonds, and triple bonds are shorter than single bonds."
Two points can also be earned in part c, one for naming the correct molecular geometry for carbon x and the other for carbon y:
- Carbon x - trigonal planar
- Carbon y - tetrahedral
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.