Unit 3 Overview: Intermolecular Forces and Properties
13 min read•june 18, 2024
Dalia Savy
AP Chemistry 🧪
269 resourcesSee Units
From the College Board
👀 Develop Your Understanding of this unit
According to the College Board, "Transformations of matter can be observed in ways that are generally categorized as either a chemical or physical change. The shapes of the particles involved and the space between them are key factors in determining the nature of physical changes.
The properties of solids, liquids, and gases reflect the relative orderliness of the arrangement of particles in those states📚, their relative freedom of motion🏃, and the nature and strength of the interactions between them💪. There is a relationship between the macroscopic properties of solids, liquids, and gases, as well as the structure of the constituent particles of those materials on the molecular and atomic scale. In subsequent units, students will explore chemical transformations of matter."
🔎 Big Idea Questions
- How do interactions between particles influence mixtures?
- Why does the smell of perfume💐 only last a short time?
- Why can you swim in water🌊 but you cannot walk through a wall🧱?
- How are the properties of gases described?
- How can you determine the structure and concentration of a chemical species in a mixture?
AP Chemistry Unit 3: Intermolecular Forces & Properties
Welcome to unit three of AP Chemistry! This unit is titled "Intermolecular Forces and Properties." In previous units, we've learned all about intramolecular forces, things like how atoms are built, orbitals, bonding, etc.
In this unit, we dive deep into what happens when you put a bunch of molecules (or atoms, but mostly molecules) together. We begin with a look at intermolecular forces and the states of matter, then move into gases and solutions. This unit is super exciting because there are tons of real-world, out-of-lab experiences you have with these concepts every day and we'll come to learn all about them.
About 18-22% of the exam is about this unit! It's the largest unit on the exam but really interesting, so let's get to it.🧠
Many students confuse intermolecular forces with intramolecular forces. The best way to remember the difference is that "inter" means between, while "intra" means within. From here, you can remember that intermolecular forces hold molecules together, while intramolecular forces hold atoms within a molecule together.
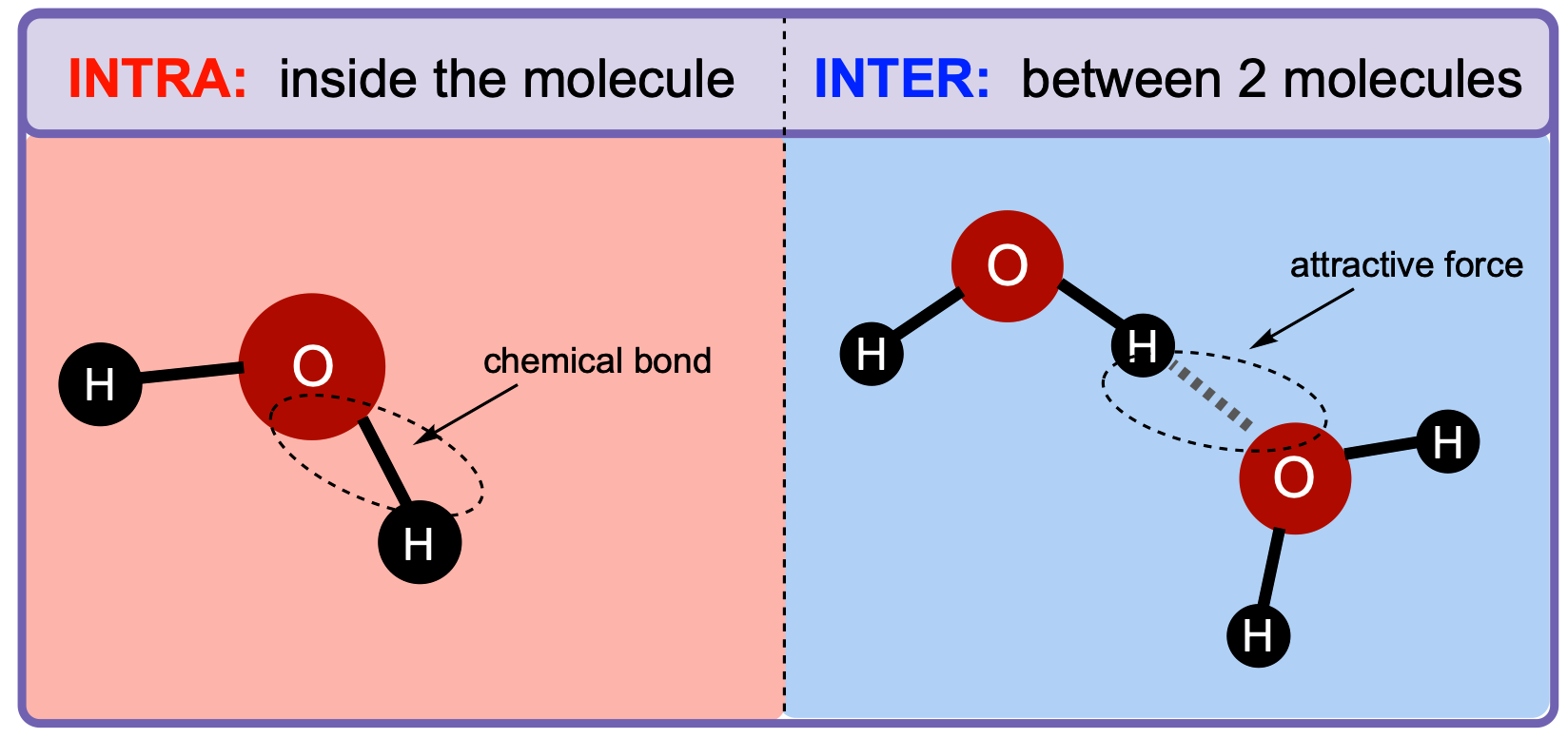
Image Courtesy of Clutch Prep
There are four major types of intermolecular forces that you are going to have to understand the relative strengths of. London Dispersion Forces (LDFs) are the weakest types of IMFs and exist in all molecular samples. They are the only forces holding together two non-polar molecules and noble gases in their solid or liquid phases. LDFs form when a molecule with a temporary dipole induces a dipole on its neighboring molecule. There is then an LDF between the partial negative side of one molecule and the partial positive side of another molecule.
Dipole-dipole interactions are a bit stronger than LDFs because they result from permanent dipoles in a sample of polar molecules. Hydrogen bonding is the strongest IMF in pure substances and occurs only when hydrogen is directly bonded to fluorine, oxygen, or nitrogen. Ion-dipole interactions are even stronger than hydrogen bonding, but only occur in a mixture of an ionic compound and polar molecules.
There are two major categories of solids: amorphous solids and crystalline solids. Amorphous solids are those that do not have a periodic crystal structure, and therefore, have a considerable disorder in their structures. Crystalline solids, on the other hand, have their particles arranged in a regularly repeating pattern.
The focus of solids in this course is on crystalline solids, such as metallic solids, ionic solids, molecular solids, and covalent network solids. Each of these has its own respective properties that you will come to understand when taking a look at their structure.
🛠3.3 Solids, Liquids, and Gases
Regardless of the type of solid, all solids retain their own shape and volume. They don’t expand to fill their container because the particles in a solid are packed very closely together and cannot move. The intermolecular forces are strong enough to keep the particles in place.
Since liquid particles aren't tightly packed together, they have the ability to flow past one another (fluidity). The intermolecular forces are strong enough to hold the molecules closely together, but not strong enough to hold them in place. Liquids tend to minimize their surface area, may experience capillary action, and have varying viscosity.
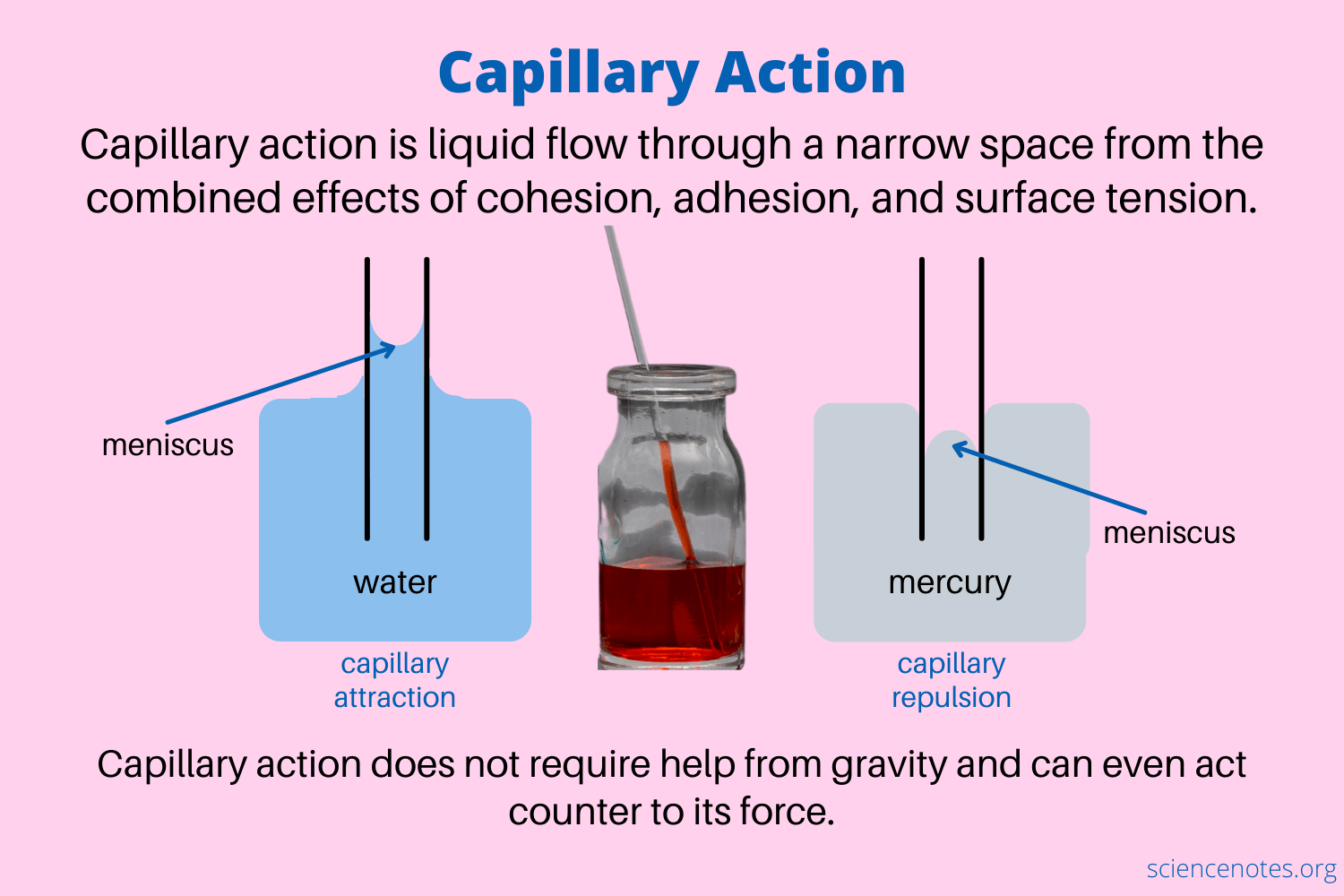
Image Courtesy of Science Notes
Gases assume the volume and shape of their container. Gas particles move rapidly in straight lines; more about the behavior of gases is covered in the rest of this unit! The molecules have enough energy to overcome any intermolecular forces that exist, allowing them to move freely. They are compressible, flow readily, and expand to fill the container.
Since gases can move freely, many chemists have attempted to explain the behavior of gases. Several gas laws are included in this course that explain the behavior of ideal gases, which are gases that follow the ideal gas law and Kinetic Molecular Theory.
The ideal gas law is basically on every AP examination: PV=nRT, where P is pressure in atm, V is volume in L, n is moles of gas, R is the universal gas constant, and T is temperature in Kelvin.
Dalton's law of partial pressures describes how the total pressure of a mixture of gases is the sum of the partial pressures of each gas. This concept is very important since most gases naturally exist in mixtures (think about the air!).
🏃♀️3.5 Kinetic Molecular Theory
The Kinetic Molecular Theory (KMT) explains the behavior of ideal gases and it has five key assumptions:
- There are no interactions between gas particles.
- Ideal gases are negligible or have no volume.
- Ideal gas particles move in random, constant, straight-line motion.
- Collisions between ideal gas particles are elastic.
- When observing particles, their kinetic energy is directly related to their velocity. All gases have the same average kinetic energy at a given temperature.
Maxwell-Boltzmann distributions display the energy at given temperatures for a gas and are based on the fifth concept of the KMT. They generally show that as temperature increases, the range of velocities becomes larger as particles move at a higher speed.
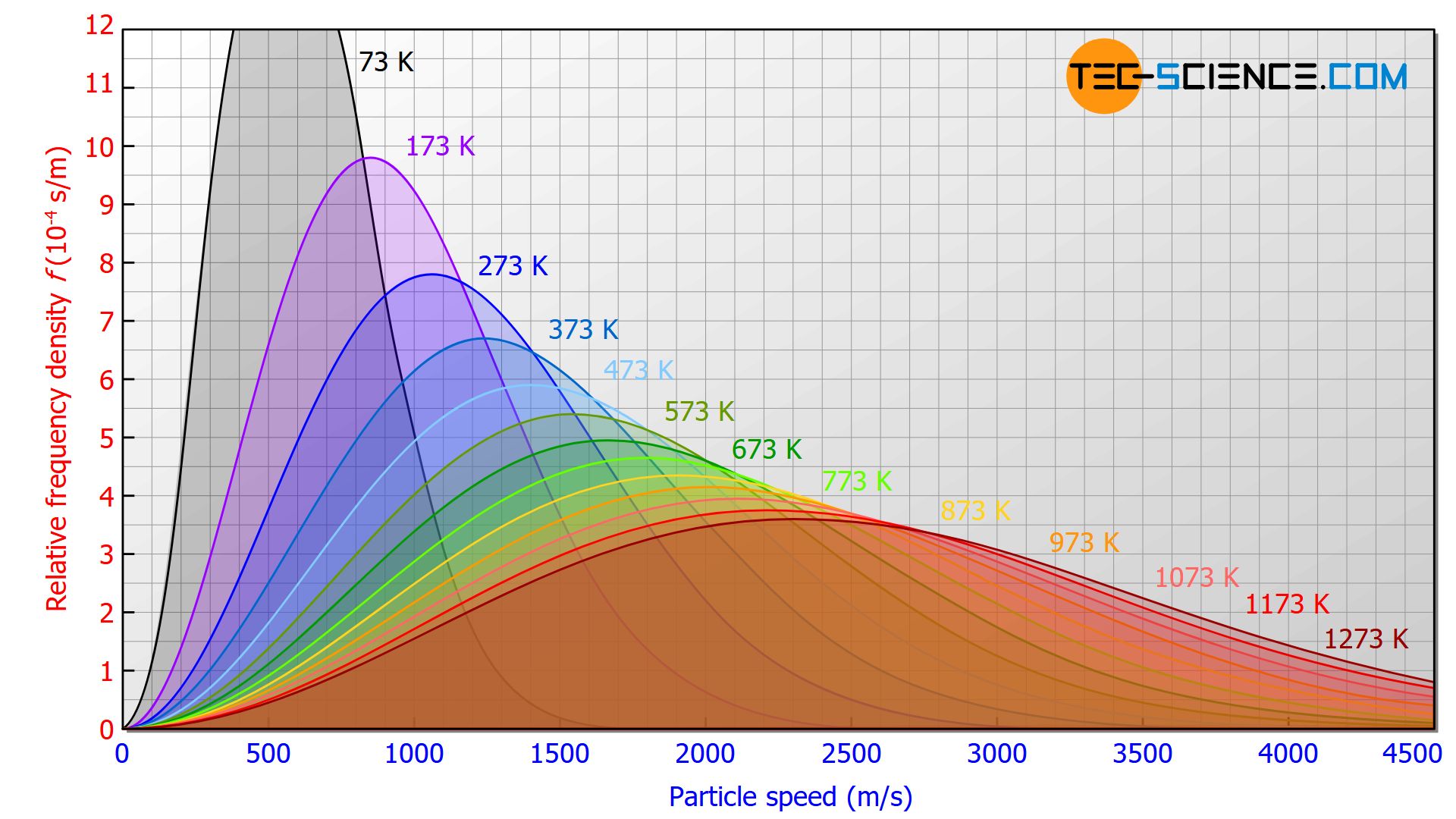
Image Courtesy of Tec-Science
🧮3.6 Deviation from Ideal Gas Law
In the real world, gases don’t always behave as defined by the kinetic molecular theory. This is because gas particles can become attracted to each other and they make up a significant portion of a gas sample's volume. Conditions of low temperatures and high pressures can cause gases to deviate from ideal gas behavior in these ways.
The Van der Waals equation makes corrections to the pressure and volume terms of the ideal gas law to represent real gases. It adds to pressure since the pressure is lower in real gases, and it subtracts from volume since volume is higher in real gases.
What does (aq) stand for when taking a look at a chemical reaction? This represents aqueous, or a substance dissolved in water. Solutions are homogeneous mixtures (uniform in composition) where the solute is uniformly distributed within the solvent. The solute is the substance that is dissolved, while the solvent is the substance that does the dissolving.
When discussing solutions, we usually refer to the concentration of a solute dissolved in a solvent. Concentration is a measure of the amount of solute that is dissolved in a given amount of solvent. Molarity (M) is a way to represent the concentration of a solution and is defined as the number of moles of solute dissolved in one liter of solvent.
Chemicals in solution are transported in high concentrations. Therefore, when completing a lab, chemists must dilute the solutions to a lower concentration. They can do this by lowering the moles of solute dissolved in the solution, or by adding solvent and increasing the volume of the solution.
We can use M1V1=M2V2 to calculate the exact amounts we need to dilute a solution to the desired concentration.
📷3.8 Representations of Solutions
Solutions can be represented with particle/molecule diagrams that show the interactions between solvents and solutes. This may be tested on the AP examination to see if you understand what a solution is made up of.
This section is a bit short, but we also discuss electrolytes. Electrolytes are substances that generate electricity in solution because of the presence of ions that can carry an electric current.
💔3.9 Separation of Solutions and Mixtures Chromatography
When dealing with solutions, you will have a solute (or multiple solutes) dissolved in a solvent. Many times, a chemist will need to separate these solutions, especially after a chemical reaction has taken place. There are many ways to separate solutes from their solvent based on physical properties and differences in their intermolecular forces.
Evaporation is a process by which a solvent is boiled, and thus evaporated, in order to separate out the solute. Filtration filters insoluble substances out of solution, leaving you with the desired filtrate.
Chromatography is a technique that separates chemical species based on their interaction with a stationary phase, which is typically a solid surface or a liquid. Paper chromatography and thin-layer chromatography are typically those to be tested on the AP Chemistry exam, but column chromatography is also used! Distillation is also used to separate solutions of liquids, but based on differences in boiling points.
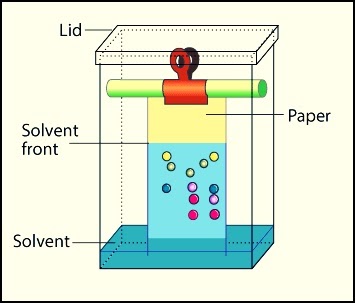
Image Courtesy of 14impressions; Paper chromatography
To put it simply, solubility is the ability of a substance to dissolve in a solvent to form a homogeneous mixture. A substance that is soluble in a solvent will dissolve completely to form a solution, while a substance that is insoluble in a solvent will not dissolve and will remain in a separate phase.
Every solution, no matter the solute and solvent, has something called a saturation point. Essentially, this is the point at which no more solute can be dissolved in the solvent. You'll learn how to interpret a solubility curve which shows the relationship between the solubility of a substance in a solvent and the temperature of that solvent.
🔬3.11 Spectroscopy and the Electromagnetic Spectrum
Light is an interesting quantum idea because of the fact that it acts both as a particle (the photon) and as a wave. This is called particle-wave duality.
When thinking of a wave, it is useful to visualize it as a sine wave, oscillating back and forth periodically. The amplitude of a wave refers to its vertical height and determines the light's brightness. Wavelength refers to the length of one period of the wave and it determines the color of the light we see. Frequency describes the number of waves that pass a fixed place in a given amount of time and it is inversely proportional to wavelength.
Visible light, which is the light we can see, is only a small category of light. The electromagnetic spectrum includes all wavelengths of electromagnetic radiation, ranging from very short gamma rays to very long radio waves. A key trend to note is that electromagnetic radiation can be characterized by its wavelength; the shorter the wavelength, the higher the frequency.
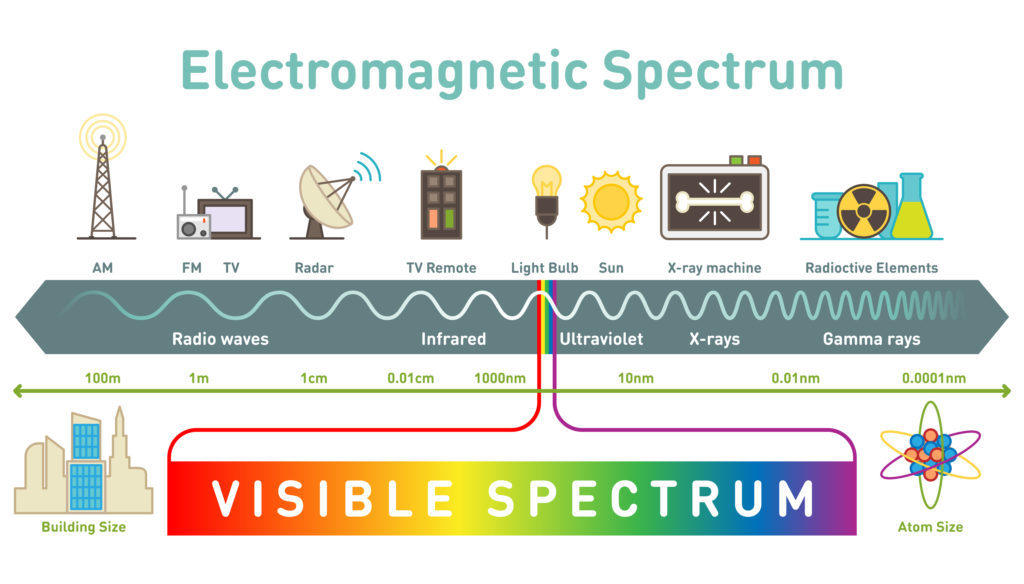
Image Courtesy of Science Sparks
Albert Einstein uncovered the photoelectric effect which showed Max Planck's theory at work. He theorized that light is emitted in discrete quantities in which energy is proportional to the frequency of the wave.
Einstein's experiments showed that electrons are ejected from the surface of certain metals when exposed to light of a minimum threshold frequency or minimum energy. The key is that this only occurs if the frequency of light reaches a certain threshold. If the light does not reach this threshold, the metal absorbs the light.
Spectrophotometry is a technique used to measure the amount of a specific substance in a sample based on its absorption of light. It involves the use of a spectrophotometer, which is an instrument that measures the amount of light absorbed by a sample at a specific wavelength or range of wavelengths.
The Beer-Lambert Law is a linear relationship between the absorption of light and the concentration of the absorbing species: A = εbc. A is the absorbance of the solution, ε is the molar absorptivity of the substance, b is the path length of the cuvette, and c is the concentration of the substance in molarity.
📝Unit 3 Key Vocabulary
Intermolecular Forces - the attractive or repulsive forces between entire molecules due to differences in charge.
London Dispersion Forces - a type of intermolecular force that arises from the fluctuating dipoles in nonpolar molecules.
Dipole-Dipole Forces - intermolecular forces that result from the attraction between the positive end of one dipole and the negative end of another dipole.
Hydrogen Bonding - a type of attractive interaction that occurs between a hydrogen atom covalently bonded to a highly electronegative atom, such as nitrogen, oxygen, or fluorine.
Polarizability - the measure of a molecule's ability to distort its electron cloud in response to an applied electric field.
Ion-Dipole Attractions - interactions between an ion and a polar molecule, in which the positive or negative charge of the ion is attracted to the partial negative or positive charge of the molecule.
Ion-Ion Attractions - the attractive forces between ions of opposite charge.
Ionic Solids - crystalline solids that are held together by the attraction between positively and negatively charged ions.
Covalent Network Solids - solids in which the atoms are held together by covalent bonds, forming a continuous network of atoms throughout the solid.
Molecular Solids - solids in which the atoms or molecules are held together by intermolecular forces, rather than by covalent or ionic bonds.
Metallic Solids - solids in which the atoms are held together by a metallic bond, which is a type of chemical bond formed by the delocalization of valence electrons over a large number of atoms.
Crystal Lattice - the three-dimensional arrangement of atoms, ions, or molecules in a crystal.
Delocalized - refers to the absence of a fixed location or specific arrangement for something, such as electrons in a metallic bond or molecular orbitals in a covalent bond.
Substitutional Alloy - an alloy in which the atoms of one element are substituted for the atoms of another element in a crystal lattice.
Interstitial Alloy - an alloy in which atoms of one element occupy the interstitial sites (empty spaces) within the crystal lattice of another element.
Compressibility - the degree to which a material can be squeezed; a measure of volume change when pressure is applied to a substance.
Fluidity - the ability of particles to flow past one another.
Surface Tension - the tendency of liquids to minimize their surface tension.
Capillary Action - the spontaneous rising of a liquid against gravity.
Viscosity - a measure of a liquid's resistance to flow due to IMF strength.
Density - the mass of a substance per unit volume.
Ideal Gas Law - an equation of state that describes the relationship between the pressure, volume, temperature, and amount of gas.
Combined Gas Law - a gas law that combines the ideal gas law with the laws of thermodynamics to describe the behavior of a gas under more general conditions.
Dalton's Law of Partial Pressures -states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas.
Mole Fraction - the ratio of the number of moles of that component to the total number of moles of all components in the mixture.
The Kinetic Molecular Theory - a theory that explains the behavior of gases in terms of the motion and collisions of the gas molecules.
Maxwell-Boltzmann Distributions - describe the probability of finding a particle with a certain velocity in a gas.
Effusion - the process by which a gas passes through a hole or small opening.
Diffusion - the process by which molecules spread out and mix as a result of their random thermal motion.
Solution - a homogeneous mixture of two or more substances.
Solute - the substance being dissolved in a solution.
Solvent - the substance in which the solute is dissolved to form a solution.
Molarity - a measure of the concentration of a solution, defined as the number of moles of solute per liter of solvent.
Electrolytes - substances that conduct electricity when dissolved in water or melted.
Filtration - a process used to separate solids from liquids or gases by passing a mixture through a filter.
Chromatography - a technique used to separate and analyze the components of a mixture.
Distillation - a process used to separate and purify liquids by vaporizing and condensing them.
Solubility - the maximum amount of a solute that can be dissolved in a solvent at a given temperature.
Saturated Solution - a solution that contains the maximum amount of solute that can be dissolved in a solvent at a given temperature.
Supersaturated Solution - a solution that contains more solute than can be dissolved in a solvent at a given temperature.
Wavelength - the distance between two consecutive crests or troughs of a wave.
Frequency - the number of waves that pass a given point in a given amount of time.
Planck's Constant - a physical constant that describes the relationship between the energy of a photon and its frequency.
Photoelectric Effect - the emission of electrons from a metal surface when it is irradiated with light or other electromagnetic radiation.
Electromagnetic Spectrum - the range of all types of electromagnetic radiation, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
Beer-Lambert Law - an empirical law that describes the absorption of light by a substance as it passes through a medium.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.