Dalia Savy
Kanya Shah
AP Chemistry 🧪
269 resourcesSee Units
What do you currently know about temperature and pressure? Let's expand on those definitions and start thinking about how gases respond to changes in pressure and temperature.
Pressure and Temperature
Gases exert pressure on their surroundings. The key phrase you want to associate with pressure is "the number of times particles hit the walls of the container." If you are ever asked to explain any of the relationships in this key topic, always mention that phrase if pressure is involved!
Below is the value for standard pressure in four different units. Standard pressure is a measure of the pressure exerted by the weight of the Earth's atmosphere at a particular location, and you may know it better as "atmospheric pressure."
Standard Pressure: 1.00 atm = 760 mm Hg = 760 torr = 101.3 kPa
Temperature is a function of mass and velocity and it is most often seen as the average kinetic energy of particles in a substance on this AP exam. Therefore, the higher the temperature of a substance, the greater the average kinetic energy of its particles. We can think of temperature as how fast particles are moving in a substance.
Standard Temperature: 0 degrees C° = 273.15 K (°C + 273.15 = K)
STP, or Standard Temperature and Pressure, are conditions at 1 atm and 273.15 K. You will often see this term on AP questions. Now let's get into it. Don't worry, we're providing some perfect responses to AP questions too🥳.
Gas Laws & Relationships
Gas laws are a set of laws that describe the relationship between pressure, volume, temperature, and moles of gas. The following relationships hold true when the amount of gas is constant.
Boyle’s Law
Boyle's law describes the relationship between the pressure and volume of an ideal gas under constant temperature. It indicates an inverse relationship between pressure and volume: P1V1 = P2V2, where P stands for pressure, V stands for volume, and the numbers refer to the initial and final pressures and volumes.
When volume decreases, the particles collide with the side of the container more often, thereby increasing pressure.
Charles’ Law
Charles' law describes the relationship between the volume and temperature of an ideal gas under constant pressure. Charles' law indicates that there is a direct relationship between volume and temperature: V1/T1 = V2/T2, where V stands for volume, T stands for temperature, and the numbers refer to the initial and final conditions.
When temperature or average kinetic energy increases, particles move faster causing more and stronger collisions with the walls of the container. The volume increases to keep the pressure constant.
Gay-Lussac's Law
Gay-Lussac’s law describes the relationship between the temperature and pressure of an ideal gas under constant volume. His law indicates a direct relationship between pressure and temperature: P1/T1 = P2/T2, where P stands for pressure, T stands for temperature, and the numbers refer to initial and final conditions.
As temperature increases, particles move faster causing collisions with the sides of the container to happen more often and to be stronger. This increases the pressure.
Avogadro's Law
Avogadro's law describes the relationship between volume and the moles of gas in the sample. His law indicates a direct relationship between these two variables: V1/n1 = V2/n2, where V stands for volume, n stands for moles of gas, and the numbers refer to initial and final conditions.
Adding more particles to a container causes more collisions with the walls of the container and the volume increases to keep the pressure constant.
Avogadro also found that equal volumes of gases at the same temperature and pressure contain the same number of particles. For example, 5 L of H and 5 L of He at STP contain the same number of particles.
The Combined Gas Law
Three of these laws can be expressed in the combined gas law: P1V1/T1 = P2V2/T2. When solving problems, you can ignore any of the variables that aren’t addressed (ex. in a pressure change problem where you find volume, ignore T and do P1V1 = P2V2 which is really Boyle's Law).
In reality, you only have to remember this equation out of the ones we learned so far but you should understand the reasons behind every relationship.
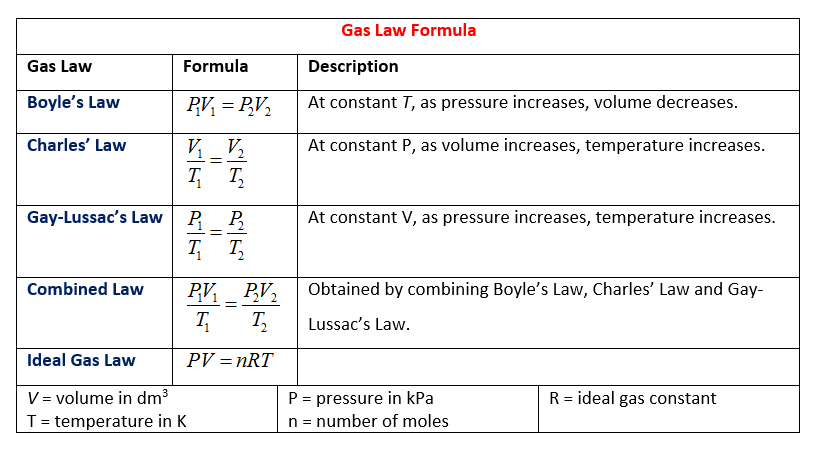
Image Courtesy of Online Math Learning
Ideal Gas Law
The last equation on that chart above is the ideal gas law: PV=nRT. The following information can be found on the AP Chemistry reference table, but it's quick and easy to memorize!
- P = pressure in atm
- V = volume in L
- n = moles of gas
- R = universal gas constant (0.08206 Latm/molK)
- T = temperature in Kelvin
In every question, we must remember to convert temperature to the Kelvin scale, volume to liters, and pressure to atm. If you ever forget which units you need for these variables, look at the given units of R on the reference sheet!
The ideal gas law is on almost every single AP Examination, so make sure you nail it. Don't worry, it just requires the plugging in of some numbers and sometimes, stoichiometry which you probably mastered by now.
Dalton's Law of Partial Pressure
"In a sample containing a mixture of ideal gases, the pressure exerted by each component (the partial pressure) is independent of the other components."
According to Dalton's law of partial pressure, the sum of all the partial pressures of each gas in a mixture of gases is equal to the total pressure. In mathematical notation, this is expressed by saying: P = Pa + Pb + Pc... where a, b, and c are different gases. You may be wondering then, how do we calculate partial pressure🤔?
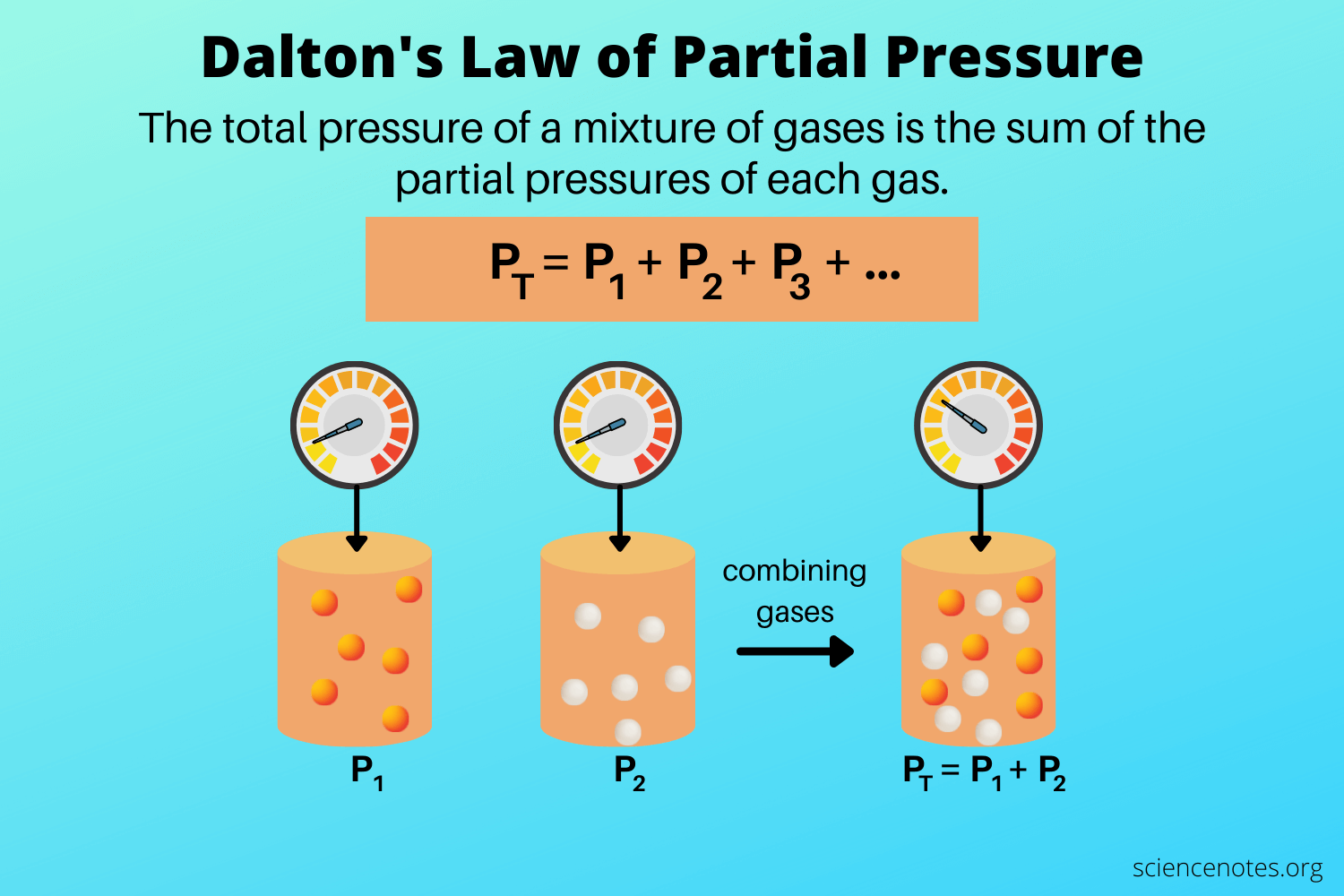
Image Courtesy of Science Notes
To do this, we use the mole fraction of that gas. Mole fraction is denoted by Xa and equals moles A/total moles. For example, if we have a mixture of 3 mol O2 and 4 mol H2, the mole fraction of O2 = 3/(3+4) = 3/7. Then, partial pressure = Xa * total pressure.
Another way to represent Dalton's Law of Partial Pressures is:
All you have to do here is plug in the values you have. Px represents partial pressure and the fraction on the right is the mole fraction itself. Memorizing the latter representation is much simpler than going through the mole fraction and then calculating pressure.
AP Chemistry Reference Table
As you progress through the course, it is good to familiarize yourself with what information is given on the reference sheet. The following section is what you are given for this chapter and the highlighted information is what you should know thus far from this unit:

Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.