7.3 Reaction Quotient and Equilibrium Constant
4 min read•june 18, 2024
Dalia Savy
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
So far, we’ve talked exclusively about reactions at equilibrium. However, reactions can be at any point! They can be pre-equilibrium, post-equilibrium, or at equilibrium. We can describe where a reaction is in the process of reaching equilibrium by calculating Q, the reaction quotient.
What is the Reaction Quotient?
Essentially, in a chemical reaction, the reaction quotient (Q) is a measure of the concentrations of the reactants and products at a specific point in time. To start explaining what Q means, let’s take a look at the formula:
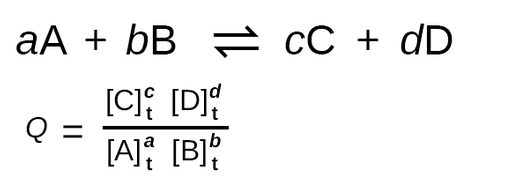
Wait wait wait 👀… back up… isn’t that the formula for the equilibrium constant K? If you thought that upon reading the formula you’d be mostly correct! However, there’s a super important distinction to be made.
As we mentioned before, when calculating with Q, the concentrations we use are concentrations at a certain time t 🕔, whereas when calculating K we must use equilibrium concentrations. Therefore, Q describes the ratio of products to reactants at any point in the reaction. By comparison, K describes the ratio of products to reactants at equilibrium specifically. By using Q we can predict whether a reaction will go further forward (produce more products), reverse (produce more reactants), or stay the same (we’re at equilibrium!). Going back to the beginning of this study guide, comparing Q and K can tell you if a reaction is in at the pre-equilibrium state, post-equilibrium state, or at equilibrium.
Q will serve to be an incredibly important concept both mathematically and qualitatively throughout the rest of unit seven. Near the latter half of the unit, you will start learning about how equilibria will react to external changes such as changes in concentration, temperature, pressures, etc. The primary justification for most of these changes (temperature being the notable exception) will be changes in Q! Q helps guide us in finding out where a reaction “is” in the process of reaching equilibrium whether we haven’t quite gotten there yet, we’re there, or if we overshot and have to go back to return.
Understanding Equilibrium vs. non-Equilibrium Concentrations
We’ve introduced the idea of concentrations not at equilibrium. These are concentrations that are either before or after equilibrium. At these concentrations, a reaction will readjust itself to equilibrium, either creating new products or creating new reactants to return to equilibrium (this is why equilibrium is called equilibrium!). All chemical reactions tend to move toward equilibrium to reach the lowest state of potential energy and be stable.
The shift that Q takes to return to K is similar to a seesaw. If there is too much weight on one side, the seesaw can add weight to the other side to balance out! This analogy can help you visualize how Q shifts back towards equilibrium by either creating products or creating reactants to shift the reaction right or left. Now let's get into the specifics.
Comparing Q and K
The most important aspect of using Q and K to solve problems is stating what direction a reaction will move. There are three scenarios we have:
- 👈 When Q > K, the concentration of the products is greater than the concentration of the reactants compared to K. When this is true, the reaction is said to be at a "post-equilibrium" state. To decrease the concentration of products, the reaction will shift to the left and produce primarily reactants.
- ⚖️ When Q = K, the ratios are the same. Thus, the reaction is at equilibrium and there will be no net change in the concentrations of the reactants or products.
- 👉 When Q < K, the concentration of the reactants is greater than the concentration of the products compared to K. This is the "pre-equilibrium" state, and to decrease the concentration of the reactants, the reaction will shift to the right. This, in turn, produces more products!

Image Courtesy of LibreTexts
Mathematical Justification
We can also understand the relationship between Q and K mathematically. We know that for A + B ⇌ C + D, Q = [C]t[D]t/[A]t[B]t.
When Q > K we know that [C]t[D]t will be too high compared to the value at K meaning it needs to decrease and [A]t[B]t must increase in order to return to equilibrium. Therefore, the reaction will use the excess C and D to create A and B until the system is at equilibrium. This thinking can also be applied to the opposite scenario where Q < K.
If Q < K that means [C]t[D]t is smaller than it should be and [A]t[B]t is larger than it should be. Thus, in order to return to equilibrium the excess reactant will react to form the product and stabilize Q to return to K. This mathematical analysis of Q matches our qualitative ‘seesaw’ analogy from before.
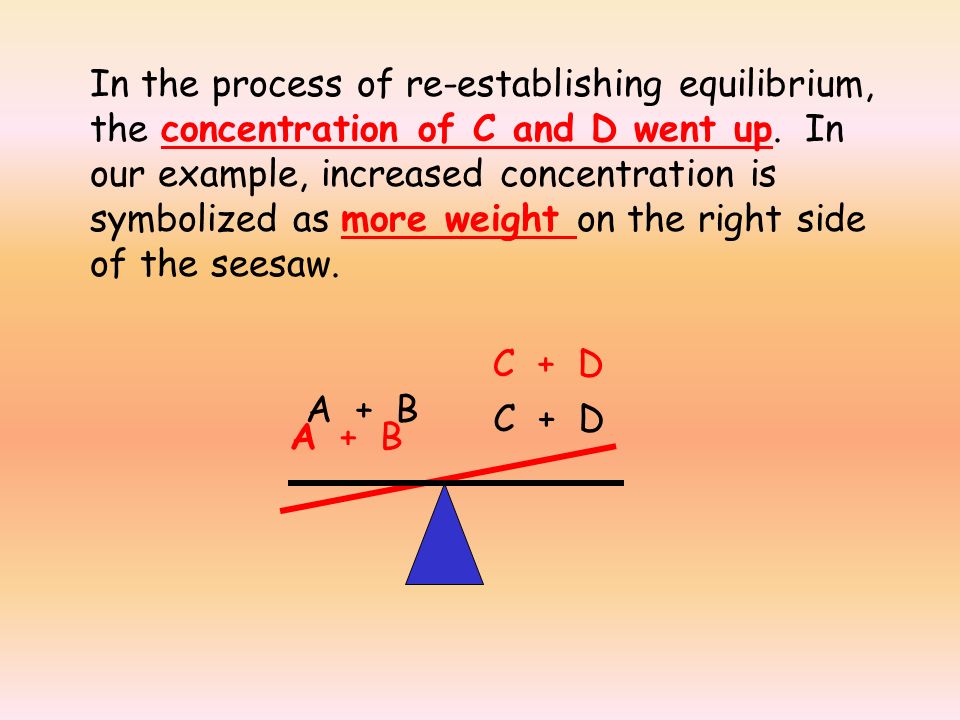
Image Courtesy of Kathlyn Parrish
Practicing Q vs. K
Consider the following reaction:
2NOBr ⇌ 2NO + Br₂
If Kc = 0.0142 and the initial concentrations are 1.0 M NOBr, 0.2M NO, and 0.8M Br₂, which way will the reaction progress to reach equilibrium?
At this point, we know that we have to calculate Q and compare it to the given Kc value of 0.0142 in order to answer the question. Let’s use Q to calculate whether or not we will move left or right:
Q = [NO]²[Br₂]/[NOBr]² = (0.2)²(0.8)/(1)² = 0.032.
0.032 > 0.0142 ⇒ Q > K ⇒ the reaction will proceed to the left because we have passed K. This goes back to the idea that, in this case, the initial concentration of the products is greater than the initial concentration of the reactants. The chemical reaction is in the post-equilibrium state and must proceed left to make more reactants and reach equilibrium. ⚖️
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.