Jillian Holbrook
AP Chemistry 🧪
269 resourcesSee Units
What are Acids and Bases?
The general population has a vague idea of what acids are; this is the same case for bases, albeit the idea for bases is a little less common.
In chemistry, an acid is a substance that has a pH of less than 7.0 when dissolved in water. Acids have a sour taste (like the acidity of a lemon) and can react with bases to form salts. They also have the ability to donate protons (H+) to other substances, which is why they are also called proton donors. 🍋
There are many different types of acids, including mineral acids (such as hydrochloric acid) and organic acids (such as acetic acid). The strength of an acid is denoted by its acidity, which is a measure of the concentration of hydrogen ions (H+) in the solution. Strong acids have higher acidity and can donate more protons than weak acids.
Bases have a pH greater than 7.0; they have a bitter taste and slippery feel (like soap). Through neutralization, bases form salts and water when combined with acids. They have the ability to accept protons (H+) from other substances, which is why they are also called proton acceptors. 🧼
Like acids, bases can be both organic and inorganic. Some examples of inorganic bases include sodium hydroxide (NaOH), also known as lye, and calcium hydroxide (Ca(OH)2). When bases dissolve in water, they accept H+ ions and make the solution alkaline (basic) in nature. Bases are important in many chemical reactions and industrial processes, such as in cleaning and sanitation products, the production of fertilizers, and certain medications.
The strength of a base is measured by its basicity, which is a measure of the concentration of hydroxide ions (OH-) in the solution. Strong bases have higher basicity, meaning that they accept more protons than weak bases.
A neutral solution has a pH of 7.0 and is considered neither acidic nor basic. Thus, it has an equal balance of H+ and OH- ions.
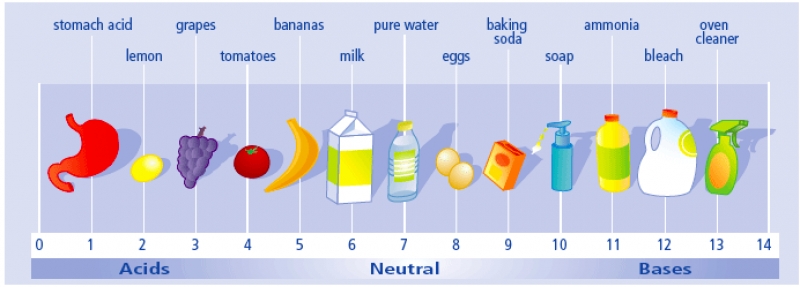
The pH scale is something you will be very familiar with by the time you finish Unit 8.
Unit 8 Overview
Acid-base chemistry essentially analyzes the complicated paths that free protons take whenever a chemical is dissolved in a solution. A free proton is a bonded hydrogen that is accessible by other chemicals, whereas a restricted proton is a proton that is guarded by other atoms in a particular compound.
In this unit, you will look at measuring concentrations of free protons (H+ ions) to find pH and pOH regarding weak acids and bases that do not dissociate fully (meaning that the equilibrium that you learned last unit will come in handy!) and strong acids and bases that do.
Once you understand pH and pOH, acid-base chemistry expands into learning about buffers (mixtures of acids and conjugate bases or bases and conjugate acids) and titrations, which you may be familiar with from Unit 4. With titrations in particular, we will be examining more complicated ways to do titrations and make calculations based on data, which is a big topic on the AP exam!
Acid-base reactions are essentially the basis for any biochemical reaction, ie. it allows life to prosper, and there are only a select handful of things more important to human beings than our own lives. Now get excited; Unit 8 is a hard unit, but also a suuuper interesting one! ✨

Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.