Dalia Savy
Kanya Shah
Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
When taking a look at a chemical reaction, there are four phases of matter you may see after each compound: (s), (l), (g), or (aq). So far in this course, we've gone over solids, liquids, and gases quite a bit. Now, let's take a look at solutions since the aq stands for aqueous, or dissolved in water!
Review of Mixtures
In AP Chemistry, a mixture is typically referring to a heterogeneous mixture in which the macroscopic properties depend upon the location of particles in the mixture. An example of a hetereogeneous mixture would be something like soil🌱, where you can actually see what is inside of the mixture, and macroscopic properties matter.
Homogeneous mixtures exist as well, but they are uniform in composition and the macroscopic properties do not vary throughout the sample. If you took a look at a homogeneous mixture, like salt water, you would not be able to see the individual parts it is made up of.
This image may help you understand the difference between a heterogeneous and homogeneous mixture:
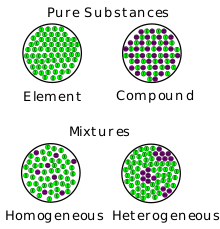
Image Courtesy of Wikipedia
What are Solutions?
Solutions are homogeneous mixtures where the particles are evenly mixed and the solute is uniformly distributed within the solvent. The solute is the substance that is dissolved, while the solvent is the substance that does the dissolving.

Image Courtesy of Pinterest
Solutions can be made up of either solids, liquids, or gases. Here are some examples that may help you connect this chemistry material with your everyday life:
Examples of Solutions
When referring to a solid solution, you should think about alloys. Alloys can be formed when two or more elements, where at least one is a metal, are in their liquid form being mixed together. When this mixture cools, the alloy is created.
- 🍳 Steel is a solid solution where the solute is carbon and the solvent is iron.
- 🎺 Brass is a solid solution where the solute is zinc and the solvent is copper.
Liquid solutions are most likely what you think of when you see the word "solution." Here are a few:
- 🧂Salt water where the solute is salt and the solvent is water.
- 🍬 Sugar water where the solute is sugar and the solvent is water.
- 🍋 Lemonade where the solutes are sugar and lemon juice and the solvent is water.
Gas solutions include:
- 💨 Air all around you.
- 🥤 Carbonated water where the solute is dissolved carbon dioxide and the solvent is water.
Within all of these examples, we can see that solutions form when one substance, the solute, disperses uniformly throughout another, the solvent.
Interactions in Solutions
Solvation is the process of a solvent dissolving a solute to form a solution. It particularly describes the attractive interaction of solvent molecules with solute particles: the molecules of the solute become surrounded by molecules of the solvent. In other words, the solute particles are said to be "solvated."
When the solvent is water, this process and interaction is called hydration. When a substance is "hydrated," it becomes surrounded by water molecules.
In essence, solutions are a kind of mixture. While solids dissolved in liquids are probably the most common type of solution, it is not the only type, and this is important to keep in mind going forward.
Representing Solution Composition
When discussing solutions, we usually refer to concentration of a solute dissolved in a solvent. Concentration is a measure of the amount of solute that is dissolved in a given amount of solvent. There are tons of ways to calculate concentration, but the most important and most commonly used form of concentration is molarity.
Molarity (M) is formally defined as the number of moles of a solute dissolved in one liter of solvent. Thus, the formula for molarity is M = moles of solute/liters of solution, hence the unit of molarity mol/L. For example, if we have a solution that contains 24 moles of HCl dissolved in 2L of water, the molarity is 24/2 = 12, or 12 M HCl as is commonly written.
Note that the numerator, moles of solute, only refers to the amount of solute. On the other hand, the denominator, volume of the solution, considers both the solute and the solvent, since they make up the solution together.
Other Ways to Represent Concentration
There are other ways of calculating concentration such as percent by mass and molality, but these won't be tested on the AP exam in May. Let's go over it anyways just in case you pursue chemistry past this course (otherwise you can completely skip this section)!
Mass percent refers to the mass of solute / mass of solution x 100. When calculating mass percent, make sure to add the mass of the solute to the mass of the solvent to get the mass of solution.
Molality (m) = moles of solute / kilograms of solvent. Pay special attention to the units used to calculate molality! It is also good to keep in mind that the numerator refers only to the solute, while the denominator refers only to the solvent (not the solution, like the last two).
Forming a Solution
There are a few steps to forming a solution and each requires a certain amount of energy:
- Expand the solute (separate the solute into individual components)
- Expand the solvent (breaking IMFs to make room for the solute)
- Solute-Solvent Interactions (forming IMFs between the solute and the solvent)
Sometimes, the first two steps can even be skipped.
Image Courtesy of Fiveable's Unit 3 Review
Diluting Solutions
Decreasing the concentration of a solute in a solution is referred to as diluting the solution. When we dilute a solution we have two options: remove some solute or add some solvent. Typically, removing solute is either completely impossible or difficult to control. Imagine having to accurately remove a certain mass of salt🧂 from a collection of salt water. It's essentially impossible! So, chemists often simply add solvent to the solution to get a lower molarity. Why does this work? Well, recall the definition of molarity: M = mol/L.
If we lower the moles of solute, of course, molarity will decrease. Conversely, if the liters of solvent increases, molarity will also decrease! Thus, adding solvent is typically the easiest way to lower the concentration of a solution.
We can calculate exact volumes using the equation: M1V1 = M2V2, where M1 and V1 are the molarity and volume of the original solution and M2 and V2 refer to the molarity and volume of the diluted solution.
Dilution is pretty important. Stock solutions used in lab are typically very concentrated since it is better to carry less material that has more substance. When its time to conduct an experiment, chemists dilute the stock solutions to the desired concentration.
How does dilution work?
M1V1 = M2V2 works because molarity * liters = mols (Moles/Liter * liter = moles) and since the number of moles remain constant throughout, M1V1 (the number of moles in the original solution) must necessarily equal M2V2 (the number of moles in the diluted solution). The only difference is in M1V1 and M2V2, molarity and volume are changing proportionally to each other.
You can just plug in the numbers and easily get any value you are solving for! 🥳
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.