AP Chemistry 🧪
269 resourcesSee Units
Multiple Choice Practice for Kinetics
Welcome to Unit 5 AP Chemistry Multiple Choice Questions! Grab some paper and a pencil 📄 to record your answers as you go. You can see how you did on the Unit 5 Practice Questions Answers and Review sheet once you're done. Don't worry, we have tons of resources available if you get stumped 😕 on a question. And if solo study is not your thing, join a group in Hours!
Not ready to take a quiz yet? Take a look at the Intro to Unit 5.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and, although they cover information outlined in the AP Chemistry Course and Exam Description, the formatting on the exam may be different.
1. When the rate of consumption of iron (II) is 15.0 M/s, what is the rate of production of the manganese (II) ion? Use the following balanced redox reaction:
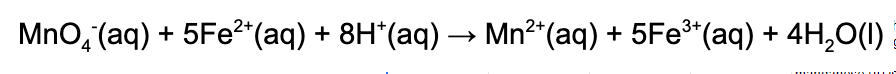
A. 3.0 M/s
B. 15.0 M/s
C. 5 M/s
D. 24.0 M/s
2. A reaction is found to have the following mechanism, what is the order of the overall reaction with respect to substance A_2?
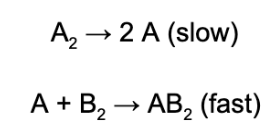
A. 0th
B. 1st
C. 2nd
D. 3rd
3. Which of the following correctly justifies why most chemical reactions slow down as they proceed?
A. As a reaction proceeds, there are less products.
B. As a reaction proceeds, there are less reactants.
C. As a reaction proceeds the temperature decreases.
D. As a reaction proceeds it consumes the catalyst.
4. A reaction is represented by the equation 2X + A -> XA + X. A plot of the concentration of A versus time, shown below, was found to have a negative slope. What is the order of the reaction with respect to A?
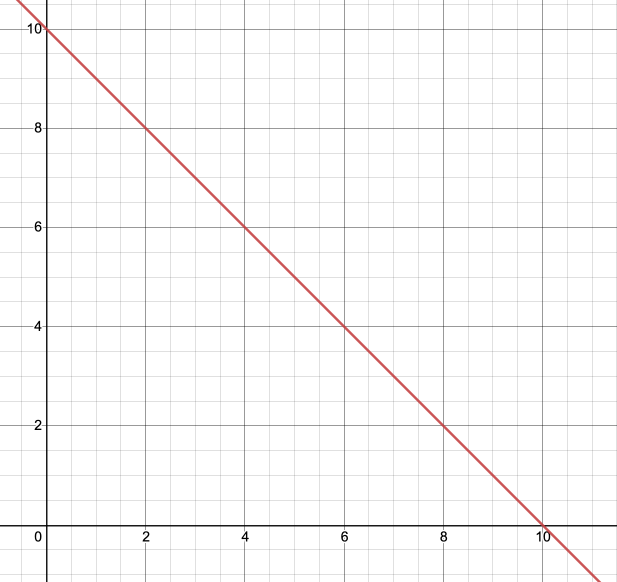
A. 0th
B. 1st
C. 2nd
D. 3rd
5. Based on the data, what is the concentration of the substance after 60 s?
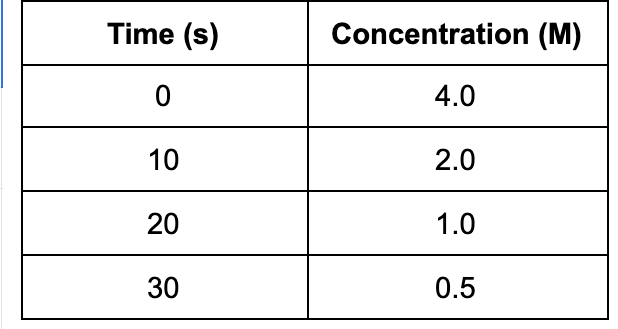
A. 0.125 M
B. 0.032 M
C. Need to know the order of the reaction.
D. 0.063 M
6. The following data table shows the concentration of a reaction and the initial rate data. Based on the table, what is the order of the reaction with respect to the reactant?
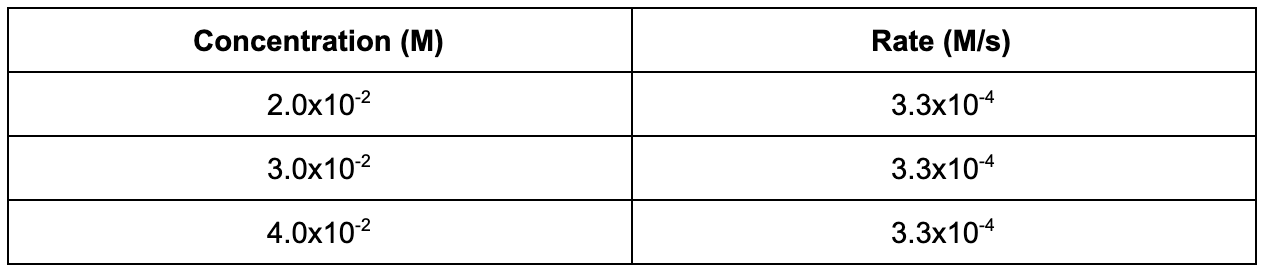
A. 0th
B. 1st
C. 2nd
D. 3rd
7. Which of the following correctly justifies why a reaction is found to occur more quickly in a solution of 1.0M HCl?
A. The acid increases the temperature of the mixture causing the rate of collision between particles to increase.
B. The acid provides an alternative pathway for the reaction which increases the activation energy.
C. The acid acts as a catalyst, providing an alternative pathway for the reaction.
D. The acid solution increases the volume of the solution, decreasing the concentration of the reactants.
8. A reaction is represented by the equation XA -> X + A. At a certain temperature, the rate constant is 2.5 1/M·s. What is the rate of the reaction if the initial [XA] = 1.1 M?
A. 2.8 M/s
B. 2.5 M/s
C. 3.0 M/s
D. Not enough information was provided.
9. Which of the following defines the collision theory?
A. A reaction will only occur if the particles collide with the right amount of energy and in the right orientation.
B. Collisions between gas particles and other particles or the wall are elastic.
C. The energy created due to the collision between atoms in mass spectrometers.
D. The minimum amount of energy required to initiate a chemical reaction.
10. Which of the following elementary reactions is first order with respect to A?
A. A+B->AB
B. AB -> A+B
C. A+A -> A_2
D. A_2 -> A+A
11. The decomposition of diamond to graphite is thermodynamically favorable. Why does this reaction not occur at an observable rate?
A. Because diamonds cannot conduct electricity while graphite can.
B. The distance between the carbon atoms in diamonds are closer than the bonds between carbon in graphite.
C. A large amount of pressure is necessary for the reaction to occur.
D. The activation energy for the reaction is extremely high.
12. The following data table shows the concentration of a reaction and the initial rate data. Based on the table, what is the order of the reaction with respect to the reactant?
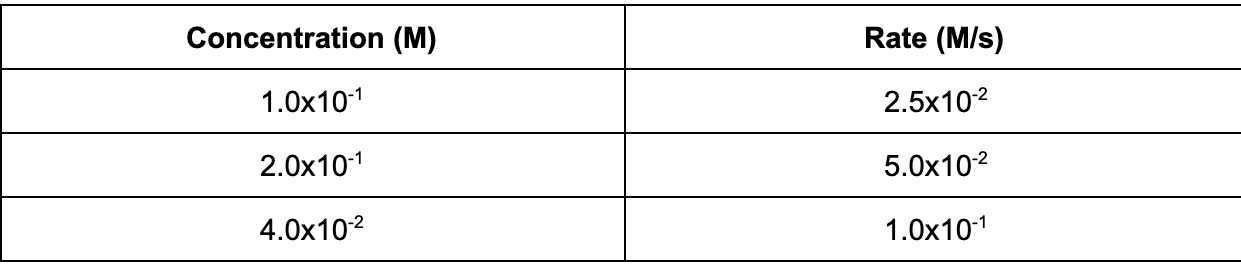
A. 2nd
B. 0th
C. 1st
D. 3rd
13. Permanganate, a purple solution, reacts with hydrogen peroxide to produce a colorless solution. Which of the following methods is used to study the rate of reaction?
A. Photospectrometry
B. Mass Spectrometry
C. Gravimetric Analysis
D. Titration
14. What is the rate law for the overall reaction represented by the following mechanism?
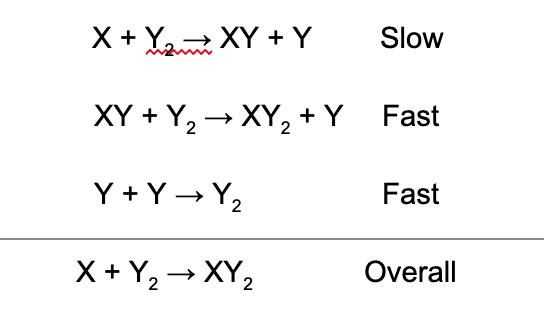
A. Rate = k[X][XY][Y]^2
B. Rate = k[X][Y_2]
C. Rate = k[XY][Y]
D. Rate = k[X]
15. Given the data, what is the rate law for the reaction X + Z -> XZ?
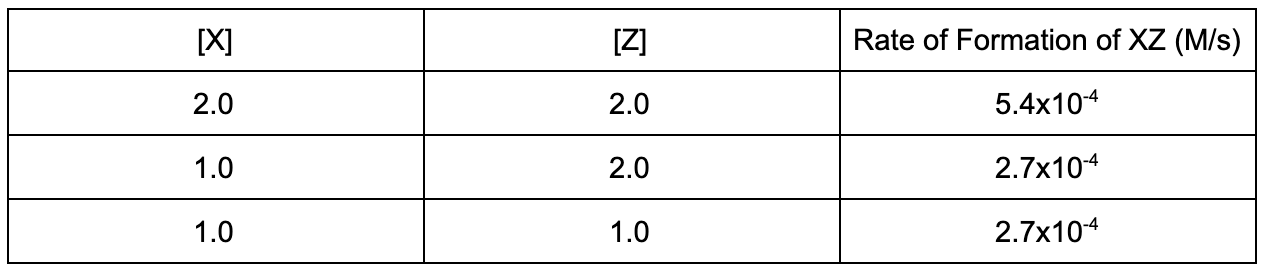
A. Rate = k[X]
B. Rate = k[Z]
C. Rate = k[X][Z]
D. Rate = k[X]^2
- 🙌 Time to check your answers on Unit 5 Practice Questions Answers and Review
- 🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.