Dalia Savy
A
Anika P
Riya Patel
AP Chemistry 🧪
269 resourcesSee Units
Introduction
Energy diagrams, also known as potential energy diagrams, can be used to represent the energy changes that occur during a chemical reaction. These diagrams show the potential energy of the reactants and products, as well as the activation energy required for the reaction to occur. The diagrams also indicate whether a reaction is endothermic or exothermic by showing the change in potential energy between the reactants and products.
The energy diagram for an exothermic reaction typically shows a decrease in potential energy as the reactants are converted to products. This indicates that energy is released by the system during the reaction.
The energy diagram for an endothermic reaction typically shows an increase in potential energy as the reactants are converted to products. This indicates that energy is absorbed by the system during the reaction.
The activation energy is represented by the highest point on the energy diagram. It is the energy required to overcome the energy barrier between the reactants and products, and it is supplied by the system or surroundings depending on the type of reaction.
Overall, energy diagrams are a useful tool for understanding the energy changes that occur during chemical reactions. They can help to visualize the potential energy of the reactants and products, the activation energy required for the reaction to occur, and whether the reaction is endothermic or exothermic.
Review
Enthalpy, represented by ΔH, is a measure of the heat energy involved in a chemical reaction. It is defined as the change in the internal energy of a system, plus the work done by the system on its surroundings. If the enthalpy of the products is lower than the enthalpy of the reactants, the reaction is exothermic and heat is released from the system. If the enthalpy of the products is higher than the enthalpy of the reactants, the reaction is endothermic and heat is absorbed by the system. Understanding the enthalpy of a reaction can help to predict the direction of a reaction, the amount of heat absorbed or released, and the potential for a reaction to be spontaneous or non-spontaneous.
Phase Changes
Phase changes can also be exothermic or endothermic. Exothermic phase changes, such as freezing, release heat energy to the surroundings as the substance changes from a liquid to a solid. Endothermic phase changes, such as melting, absorb heat energy from the surroundings as the substance changes from a solid to a liquid. Similarly, exothermic phase changes, such as condensation, release heat energy to the surroundings as the substance changes from a gas to a liquid. Endothermic phase changes, such as evaporation, absorb heat energy from the surroundings as the substance changes from a liquid to a gas. Physical or chemical processes can be described through energy diagrams, which show the change in potential energy of the substance as it changes phase and the heat absorbed or released during the process.
It's important to note that during phase changes, the energy that is either absorbed or released is known as latent heat which is heat energy that is absorbed or released by a substance during a phase change, without a change in temperature.
Melting
The process of melting, H2O(s) →H2O(l) is an endothermic process. The energy is absorbed by the system in the form of heat to change the solid phase of water (ice) to the liquid phase of water. So, the heat is going into the system to raise the temperature of the ice to its melting point, which is 0 °C (273 K) at atmospheric pressure. This process requires heat energy and the system is absorbing it, so it is endothermic.
Condensation
The process of condensation, H2O(g) → H2O(l), is an exothermic process. The energy is released by the system in the form of heat to change the gas phase of water to the liquid phase of water. So, the heat is released by the system as the temperature of the water vapor decrease to its condensation point, which is dependent on the pressure and temperature. The process releases heat energy and the system is releasing it, so it is exothermic.
To summarize, condensation is the opposite of boiling, and boiling is an endothermic process that requires energy/heat to happen. Therefore, condensation is an exothermic process that releases energy (from the system out to the surroundings).
Overview
| Phase Change | Reaction | Process |
| Melting | Solid → Liquid | Endothermic |
| Vaporization / Boiling | Liquid → Gas | Endothermic |
| Sublimation | Solid → Gas | Endothermic |
| Condensation | Gas → Liquid | Exothermic |
| Freezing | Liquid → Solid | Exothermic |
| Deposition | Gas → Solid | Exothermic |
Energy Diagrams
Energy diagrams are graphical representations that show the changes in energy that occur during a chemical or physical process. They are often used to illustrate the energy changes that occur during a reaction or phase change.
In an energy diagram, the vertical axis represents the energy, and the horizontal axis represents the progress of the reaction or phase change. The energy diagram for an endothermic process will have an upward slope, indicating that energy is being absorbed by the system as the reaction or phase change proceeds. The energy diagram for an exothermic process will have a downward slope, indicating that energy is being released by the system as the reaction or phase change proceeds.
It's important to note that the energy diagrams are theoretical tools, they are not always real representation of the energy changes during the process. Also, it's important to remember that the energy diagrams show the changes in the internal energy of the system and not the heat or work interactions with the surroundings.
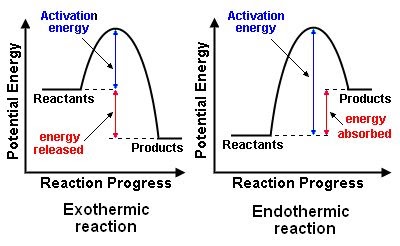
Image Courtesy of Pinterest
- PEreactants: refers to the potential energy of the reactants at the beginning of the reaction before any energy is added or released. PEproducts: refers to the potential energy of the products at the end of the reaction after all the energy has been added or released.
- Activation Energy: is the minimum amount of energy required to initiate a chemical reaction. It represents the amount of energy required to overcome the initial barriers that prevent the reactants from reacting.
- Activated Complex: is a hypothetical intermediate state that a molecule must pass through before it can react. It is the highest point on the energy diagram, representing the most unstable state of the reactants before they transform into products. The reaction occurs when the energy supplied to the reactants exceeds the activation energy and the system reaches the activated complex.
Exothermic Reactions
In the energy diagram for an exothermic reaction, the potential energy of the products is lower than that of the reactants. This indicates that energy has been released from the system, as the reactants have converted to products.
The activation energy is also lower, which means that less energy is required to initiate the reaction. The energy required to overcome the activation energy is supplied by the system, which results in the release of energy in the form of heat.
The energy difference between the reactants and the products is represented by the change in enthalpy (ΔH). In an exothermic reaction, the difference is negative, which means that energy has been released by the system.
Overall, in an exothermic reaction, the energy of the products is lower than the reactants, which means that energy has been released by the system, and the reaction is exothermic.
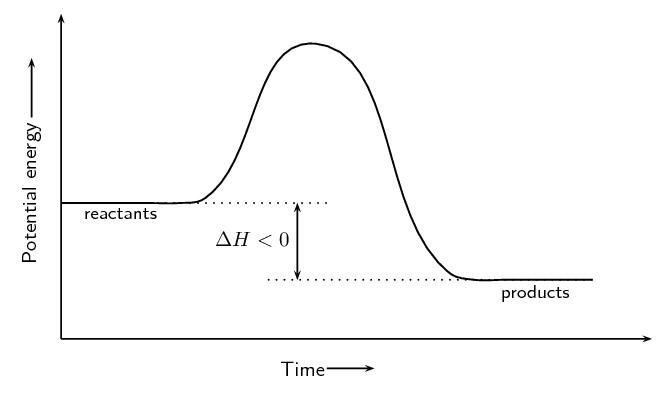
Image Courtesy of SilaVula
Endothermic Reactions
In the energy diagram for an endothermic reaction, the potential energy of the products is higher than that of the reactants. This indicates that energy has been absorbed by the system, as the reactants have converted to products.
The activation energy is also higher, which means that more energy is required to initiate the reaction. The energy required to overcome the activation energy is supplied by the surroundings, which results in the absorption of energy in the form of heat.
The energy difference between the reactants and the products is represented by the change in enthalpy (ΔH). In an endothermic reaction, the difference is positive, which means that energy has been absorbed by the system.
Overall, in an endothermic reaction, the energy of the products is higher than the reactants, which means that energy has been absorbed by the system, and the reaction is endothermic.
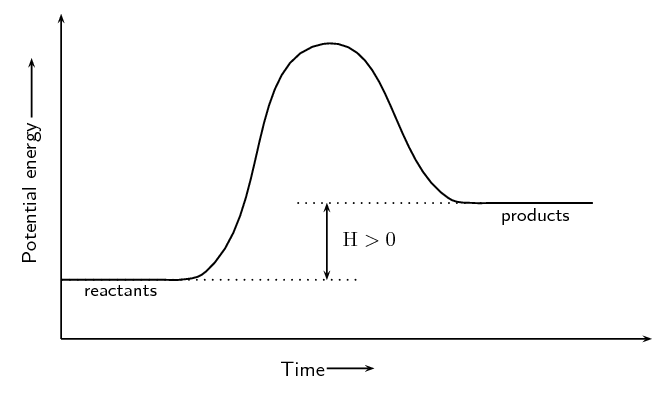
Image Courtesy of SilaVula
Now that you've seen the graph and been introduced to a few terms, let's put the two together and do a practice problem.
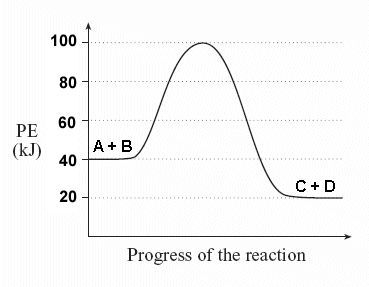
Image Courtesy of Socratic
(a) What is the potential energy of the reactants?
Just look at the y-axis for the reactants: 40 kJ!
(b) What is the potential energy of the products?
Similar to part a, just look at the y-axis for the products: 20 kJ.
(c) What is the value of ΔH?
ΔH is the difference between the potential energy of the products and the potential energy of the reactants.
Simply do: (PEproducts) - (PEreactants)
(20 kJ) - (40 kJ) = -20 kJ
In other words, 20 kJ of energy is released during this reaction.
(d) What is the activation energy?
To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point).
(energy of activation complex) - (PEreactants)
(100 kJ) - (40 kJ) = 60 kJ
In other words, it takes 60 kJ of energy to complete the reaction.
(e) Is this an endothermic or exothermic reaction?
Since ΔH is negative and the potential energy of the products is lower than that of the reactants, this is an exothermic reaction.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.