6.6 Introduction to Enthalpy of Reaction
6 min read•june 18, 2024
Dalia Savy
A
Anika P
Riya Patel
AP Chemistry 🧪
269 resourcesSee Units
Review of What Enthalpy Is
Enthalpy (H) is a measure of the total internal energy of a system, including the energy required to change the temperature and the pressure of the system. It is often used to describe heat energy changes in chemical reactions as well as phase transitions. ΔH is the change in enthalpy, which is the difference between the final and initial enthalpy of a system, and it can be measured through experimentation.
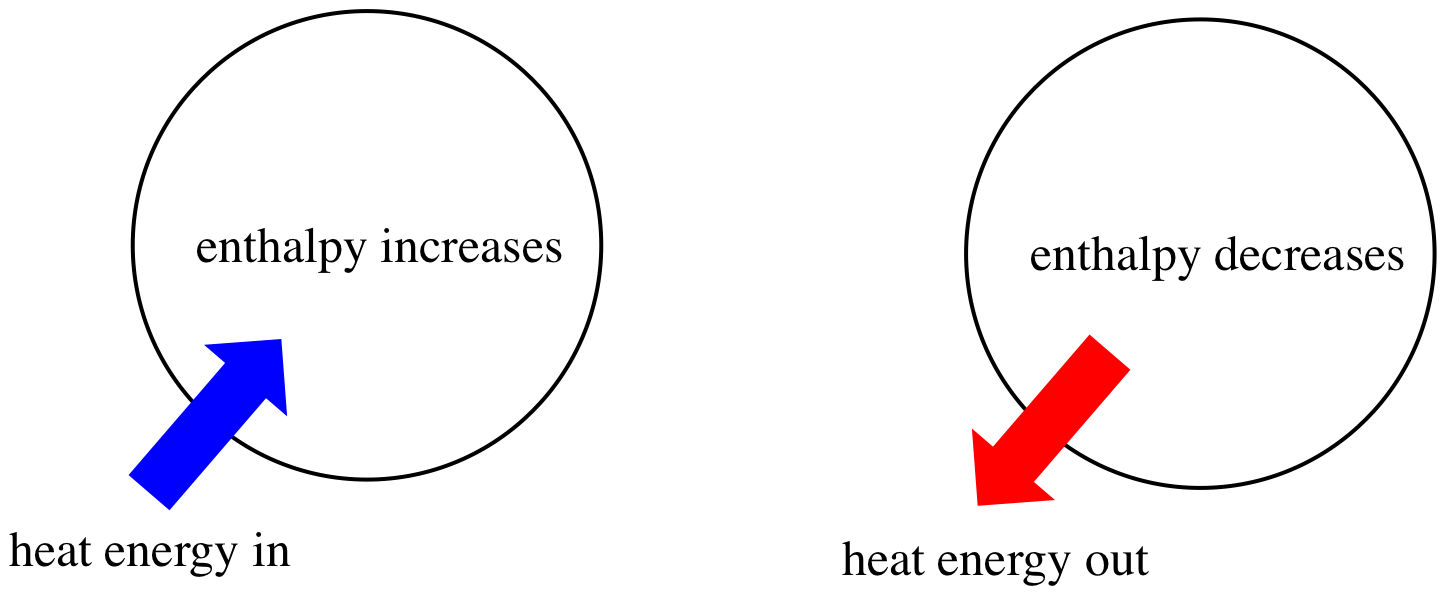
Image Courtesy of Chemistry LibreTexts
Reaction Energy, AKA: Enthalpy of Reaction
In exothermic reactions, the enthalpy of reaction (ΔH) is negative, indicating that heat is being released by the system during the reaction. In endothermic reactions, ΔH is positive, indicating that heat is being absorbed by the system during the reaction.
It's also worth noting that the enthalpy change of a reaction can also be used to predict the feasibility of a reaction. Reactions with a negative ΔH (exothermic) are thermodynamically favorable, while those with a positive ΔH (endothermic) are thermodynamically unfavorable. However, this is not the only factor that determines the feasibility of a reaction and is dependent on the Gibbs free energy change of the reaction (ΔG) and the equilibrium constant (Kc)
Examples
Here are a couple examples of exothermic and endothermic reactions:
- Exothermic: Combustion of propane (C3H8) to form carbon dioxide (CO2) and water (H2O): C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) The enthalpy change for this reaction, ΔH, is negative, indicating that heat is being released by the system during the reaction, this can be observed as a flame or an increase in temperature around the reaction.
- Endothermic: Dissolution of anhydrous copper sulfate (CuSO4) in water: CuSO4(s) + H2O(l) → CuSO4(aq) The enthalpy change for this reaction, ΔH, is positive, indicating that heat is being absorbed by the system during the reaction, this can be observed as a decrease in temperature around the reaction.
- Exothermic: Combustion of methane (CH4) to form carbon dioxide (CO2) and water (H2O): CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) The enthalpy change for this reaction, ΔH, is negative, indicating that heat is being released by the system during the reaction, this can be observed as a flame or an increase in temperature around the reaction.
- Endothermic: Synthesis of ammonia (NH3) from nitrogen (N2) and hydrogen (H2) N2(g) + 3H2(g) → 2NH3(g) The enthalpy change for this reaction, ΔH, is positive, indicating that heat is being absorbed by the system during the reaction, this can be observed as a decrease in temperature around the reaction.
- Exothermic: Neutralization of hydrochloric acid (HCl) with sodium hydroxide (NaOH) HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) The enthalpy change for this reaction, ΔH, is negative, indicating that heat is being released by the system during the reaction, this can be observed as increase in temperature around the reaction.
- Endothermic: Melting of ice (H2O) at 0°C H2O(s) → H2O(l) The enthalpy change for this reaction, ΔH, is positive, indicating that heat is being absorbed by the system during the reaction, this can be observed as a decrease in temperature around the reaction.
Negative ΔH vs. Positive ΔH
The sign of ΔH is determined by the direction of heat flow in a reaction. If heat is flowing into the system (from the surroundings), the reaction is endothermic, and ΔH is positive. If heat is flowing out of the system (into the surroundings), the reaction is exothermic, and ΔH is negative.
It's also important to note that the heat flow is not necessarily a direct measurement of temperature change, but rather it is a measure of the total energy flow into or out of the system during a reaction. As you mentioned, the endothermic reaction may cause the temperature of the surroundings to decrease, and the exothermic reaction may cause the temperature of the surroundings to increase, but this is not always the case.
In addition, ΔH doesn't take into account the fact that at different temperatures, different amounts of heat are required to raise the temperature of a substance by a given amount. The enthalpy change also does not depend on the initial and final states of the system, but only on the difference between the initial and final state.
Examples
Here are a couple of examples of reactions with negative and positive enthalpy changes:
- Combustion of Methane (CH4) to form Carbon Dioxide (CO2) and Water (H2O): CH4(g) + 2O2(g) --> CO2(g) + 2H2O(g) The enthalpy change for this reaction is negative, meaning the reaction is exothermic and releases heat into the surroundings. This can be observed as a flame or an increase in temperature around the reaction.
- Dissolution of Ammonium Nitrate (NH4NO3) in Water: NH4NO3(s) + H2O(l) --> NH4NO3(aq) The enthalpy change for this reaction is positive, meaning the reaction is endothermic and absorbs heat from the surroundings. This can be observed as a decrease in temperature around the reaction.
Note that these are simplified examples and actual values may vary depending on the conditions of the reactions.
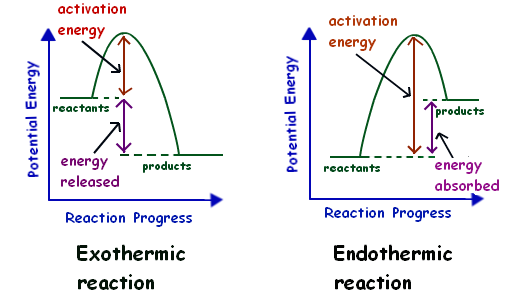
Image Courtesy of Mr Lowe
Other Terms to be Familiar with
Energy is the ability to do work or transfer heat. It is a scalar quantity and is typically measured in units of joules (J) or calories (cal). There are many forms of energy, such as kinetic energy, potential energy, thermal energy, and electromagnetic energy, among others. Energy can be converted from one form to another, but the total amount of energy in a closed system remains constant (conservation of energy).
Internal energy (E) is the sum of all the kinetic and potential energies within a system. It is a state function, which means that it depends only on the current state of the system, and not on the path taken to reach that state. The change in internal energy (ΔE) is the difference between the final and initial internal energy of a system, and it can be calculated as the heat added to or removed from the system (q) plus the work done on or by the system (w). ΔE = q + w.
Heat (q) is the transfer of energy due to a temperature difference between two objects. It is a form of energy transfer and it flows from a higher-temperature object to a lower-temperature object. It is measured in joules and it is also a scalar quantity.
Work (w) is the transfer of energy due to a force acting over a distance. It is measured in joules and it is also a scalar quantity. It's associated with the compression and expansion of a gas. Positive work is done when a gas is compressed, and negative work is done when a gas is expanded. The work done by a gas can be calculated using the formula w = -PΔV, where P is the pressure and ΔV is the change in volume.
Quick Summary
- Energy: capacity to do work or produce heat, measured in joules
- Internal energy: sum of kinetic and potential energies within a system, measured in joules, state function
- Heat: transfer of energy due to a temperature difference, measured in joules
- Work: transfer of energy due to a force acting over a distance, measured in joules, associated with compression and expansion of a gas.
Examples
Here are a few examples of how energy, enthalpy, heat, and work can be applied in different scenarios:
- A pot of water is heated on a stove. In this case, heat is being transferred from the stove to the pot of water. The heat energy causes the temperature of the water to increase, and this is an example of a heat transfer.
- A person lifts a weight in a gym. In this case, work is being done by the person as they exert a force on the weight over a distance (lifting it up). The work done is equal to the force applied multiplied by the distance over which it is applied.
- A chemical reaction occurs in a test tube. The enthalpy of the reaction can be measured by measuring the heat absorbed or released by the reaction. If the reaction is exothermic, heat will be released and the enthalpy of the reaction will be negative. If the reaction is endothermic, heat will be absorbed and the enthalpy of the reaction will be positive.
- A gas is compressed in a cylinder. As the gas is compressed, the volume decreases, and work is done on the gas by the piston. The energy of the system (the gas) increases as a result of the compression.
- A car engine converts the chemical energy of fuel into mechanical energy to power the car. The engine also generates heat as a byproduct of the energy conversion process. The heat can be used to warm the car's interior or to power auxiliary systems such as the air conditioning.
On the AP Chemistry exam, enthalpy and internal energy are synonymous terms. Same goes with bond energy and bond enthalpy, which we will review in the next key topic!
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.