Daniella Garcia-Loos
S
Saarah Hasan
AP Physics 2 🧲
61 resourcesSee Units
We all know the rule: matter can’t be created nor destroyed. But matter can undergo changes in form. Natural radioactive decay is one example of a nuclear reaction that showcases this. Other examples include nuclear fission and nuclear fusion. In all cases of nuclear reactions, nucleon number and charge must be conserved.
Radioactive Decay
For multiple reasons, certain isotopes aren’t stable. These radioactive isotopes go through a spontaneous breakdown of an unstable atomic nucleus with the emission of particles and rays. Without the correct arrangement of neutrons and protons, the nucleus can’t support itself. Pieces of the nucleus break off, changing the material.
The half-life is the time for half of a radioactive sample to decay. The decaying nuclide is known as the parent, and the resulting nuclide is known as the daughter.
Alpha Decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle. It is one of three types of radioactive decay, along with beta decay and gamma decay.
Here are some key points about alpha decay:
- Alpha decay is a type of radioactive decay that occurs when an atomic nucleus emits an alpha particle. It is one of three types of radioactive decay, along with beta decay and gamma decay.
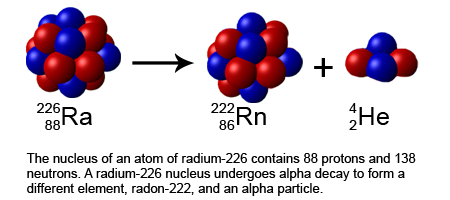
- An alpha particle is a type of particle that is made up of two protons and two neutrons, which are bound together. It is the same as the nucleus of a helium-4 atom.
- Alpha decay is a process by which an atomic nucleus releases energy by emitting an alpha particle. The alpha particle is emitted from the nucleus and is accompanied by a high-energy photon, or gamma ray.
- Alpha decay is caused by a change in the ratio of protons to neutrons in the nucleus. When the ratio of protons to neutrons is unstable, the nucleus can release energy by emitting an alpha particle. This process is called alpha decay.
- Alpha decay is accompanied by a change in the atomic number and mass number of the nucleus. The atomic number is decreased by two and the mass number is decreased by four.
When alpha decay occurs, an alpha particle-which consists of two protons and two neutrons and is the same as the nucleus of a helium-4 atom-is emitted. An alpha particle can be represented by:
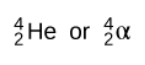
Taken from Wikimedia Commons
Beta Decay
Beta decay is a type of radioactive decay in which an atomic nucleus emits an electron or a positron. It is one of three types of radioactive decay, along with alpha decay and gamma decay.
Here are some key points about beta decay:
- Beta decay is a type of radioactive decay that occurs when an atomic nucleus emits an electron or a positron. It is one of three types of radioactive decay, along with alpha decay and gamma decay.
- Beta decay is a process by which an atomic nucleus releases energy by emitting an electron or a positron. The electron or positron is emitted from the nucleus and is accompanied by a neutrino or an antineutrino.
- Beta decay is caused by a change in the ratio of protons to neutrons in the nucleus. When the ratio of protons to neutrons is unstable, the nucleus can release energy by emitting an electron or a positron. This process is called beta decay.
- Beta decay is accompanied by a change in the atomic number of the nucleus. The atomic number is increased by one for beta-minus decay (emission of an electron) and decreased by one for beta-plus decay (emission of a positron). The mass number of the nucleus remains the same.
There are 3 subcategories of beta decay: B^-B−, and electron capture(EC).
B−Decay
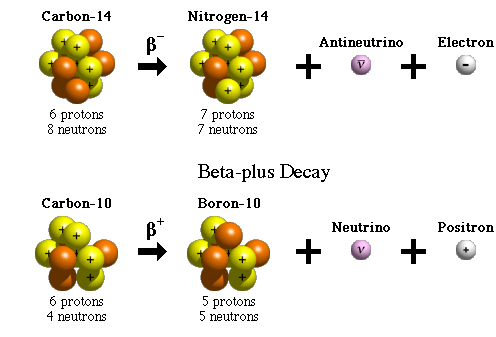
B− decay happens when a neutron turns into a proton and releases an electron, the beta particle, because the neutron-to-proton ratio is too large. The neutron transforming into a proton and an electron (plus a particle called the electron-antineutrino, ̅ν_eνe) is caused by the action of the weak nuclear force, another one of nature’s fundamental forces like the strong nuclear force.
An example of a nuclide that undergoes B−decay is carbon-14, as shown below.
Taken from Wikimedia Commons
B^+B+ Decay
A proton is transformed into a neutron and a positron, e^+, (the electron’s antiparticle) plus the electron-neutrino, v_eve, when the neutron-to-proton ratio is too small. An example of B^+ decay is carbon-12, as shown above.
Electron Capture
Another way a nucleus can increase its neutron-to-proton ratio if it’s too small is to capture an orbiting electron(e^-), and then turn a proton into a neutron.
Gamma Decay
In alpha and beta decay, the daughter was a different element than the parent. With gamma decay, however, the nucleus relaxes and sheds energy; the element doesn’t change. For the excited nucleus to drop to its ground state, it emits a photon of energy, a gamma ray(γ).
Here are some key points about gamma decay:
- Gamma decay is a type of radioactive decay that occurs when an atomic nucleus emits high-energy photons, or gamma rays. It is one of three types of radioactive decay, along with alpha decay and beta decay.
- Gamma decay is a process by which an atomic nucleus releases energy by emitting high-energy photons. These photons have very high frequency and very short wavelengths, and are similar to X-rays in many ways.
- Gamma decay is caused by a change in the energy levels of the nucleus. When an atomic nucleus is in an excited state, it can release energy by emitting a gamma ray. This process is called de-excitation.
- Gamma decay is not accompanied by a change in the atomic number or mass number of the nucleus. This means that the element does not change during gamma decay.
- Gamma decay is a random process and is not affected by external factors such as temperature or pressure. It is governed by the laws of quantum mechanics.
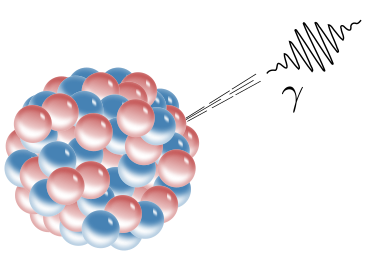
Taken from Wikimedia Commons
Let's summarize:
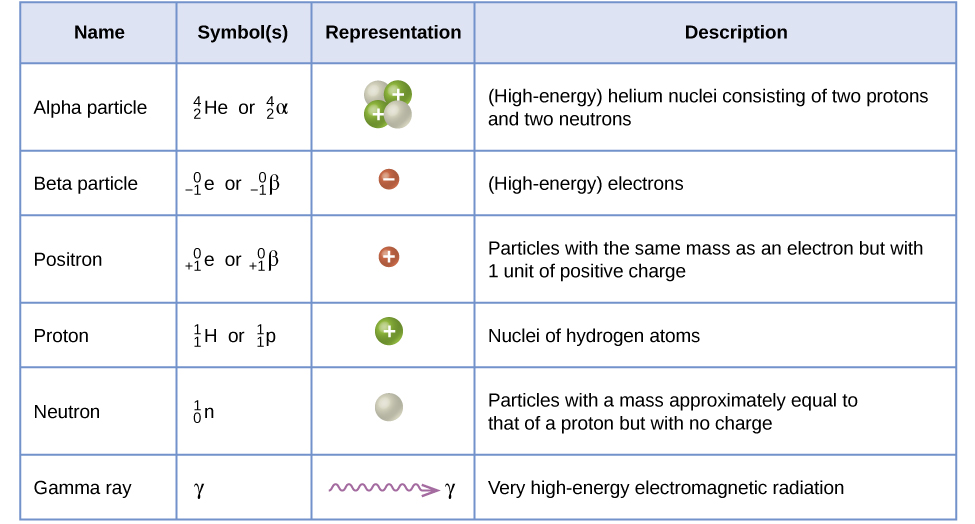
Taken from Libretexts
Practice Problems:🧩
1. An atomic mass unit is approximately equal to the mass of a(n)
A) alpha particle
B) electron
C) photon
D) positron
E) proton
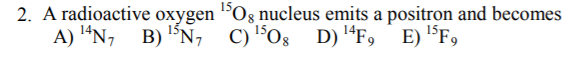
3. An alpha particle is the same as
A) a helium nucleus
B) a positron
C) an electron
D) a high energy photon
E) a deuteron
4. During a particular kind of radioactive decay, a particle is emitted from the nucleus of an atom and the atom’s atomic number increases by one. This decay necessarily involves the emission of _________ from the nucleus.
A) an alpha particle
B) a beta particle
C) a gamma ray
D) a proton
E) a neutron
5. When a radioactive nucleus emits a gamma-ray the number of
A) protons increases by one while the number of neutrons decreases by one.
B) protons decrease by one while the number of neutrons increases by one.
C) protons and neutrons each decrease by two
D) protons and neutrons each increase by two
E) protons and neutrons remain unchanged
Answers:
- E: 1 u = $1.66x10^–$$^2$$^7$ is 1/12 of carbon 12 and is approximately the same as a proton mass.
- B: For a positron to be emitted, the oxygen must have undergone beta+ decay, which is the opposite of beta– decay. In beta+ decay a proton turns into a neutron + the emitted beta particle. The mass number stays the same (P+N still the same), but the atomic number goes down by 1 since there is 1 less proton.
- A: Definition of alpha particle.
- B: This is the definition of Beta^– decay
- E: Gamma emission is pure energy so no particles change
Browse Study Guides By Unit
💧Unit 1 – Fluids
🔥Unit 2 – Thermodynamics
⚡️Unit 3 – Electric Force, Field, & Potential
💡Unit 4 – Electric Circuits
🧲Unit 5 – Magnetism & Electromagnetic Induction
🔍Unit 6 – Geometric & Physical Optics
⚛️Unit 7 – Quantum, Atomic, & Nuclear Physics
📆Big Reviews: Finals & Exam Prep
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.