Dylan Black
AP Chemistry 🧪
269 resourcesSee Units
In the last section, we introduced the idea of entropy as a measure of disorder in a system. Unlike enthalpy, which was discussed in unit 5, entropy can be measured both absolutely and as a change. This differs from enthalpy, which can only be represented as a change (we can never find H° for a reaction but we can find ΔH°).
Comparing S° and ΔS°
Entropy can be thought of both in its absolute form (S°) and as representing a change in entropy (ΔS°) (note that the ° symbol in each simply means that we are at standard conditions, that is 1atm of pressure and 273K). Absolute entropies, also known as standard entropies, describe the number of possible states a molecule can take, a measure of its disorder. Calculating these values is incredibly complex, but you will be given any absolute entropies you need on the exam. Similarly, most if not all chemistry textbooks have tables of thermodynamic data in the back of the book with a chart of standard entropies. These tables may also contain other data such as enthalpies of formation like the one below. For now, lets look at the far right column that says S°.
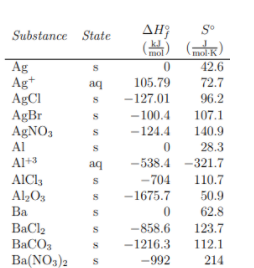
Change in entropy (ΔS°) can be thought of the same way we thought about changes in enthalpy (ΔH°) in unit 6 just thinking about entropy instead of heat changes. In Unit 6, we learned about Hess’s Law, which told us that enthalpy was a state function, meaning enthalpy changes are pathway independent. The same is true about entropy.
State Functions
Let’s take a quick side note to explain what a state function means and why it’s important. In essence, a state function is a function that has the property of pathway independence. This means that whatever “path” you take to get to the end result, the end result will be exactly the same. An example of a state function is the change in altitude when climbing a mountain, whereas an example of a non-state function is the distance traveled. Since no matter which way you go, you will end up at the top of the mountain, your change in altitude will always be the altitude. Whether you went straight up, zigzagged, went curvily, or any other pathway, your change in altitude will be the same at the end. This makes this function pathway independent. On the other hand, the distance you travel up the mountain does depend on the path you take. If you go straight up, the distance will differ from if you zigzag and for any other path you take. Therefore, the distance traveled is not a state function and is not pathway independent.
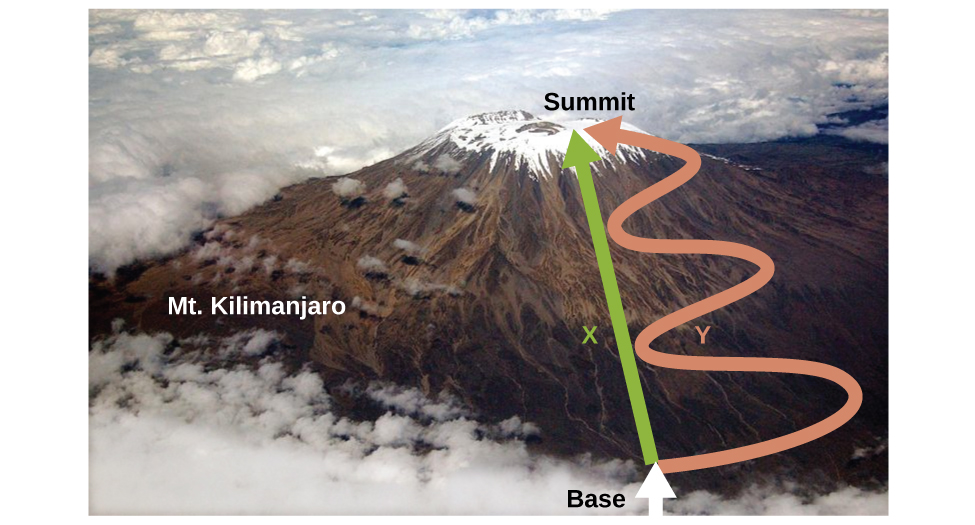
Image From Chemistry Stack Exchange
Applying the idea of a state function to entropy, we can logically find the following equation (note that it is strikingly similar to an equivalent statement about enthalpy change):
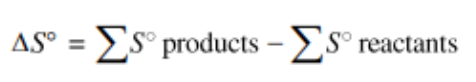
This equation tells us that for a reaction the overall entropy change is the sum of the changes in the entropies of the products minus the sum of the entropies in the reactants. This can be applied the same way we did in unit 5 with enthalpy, so you can simply use the table and plug in numbers for each product/reactant.
🔥 Pro Tip: Remember your stoichiometric coefficients! The real equation has you multiplying by the stoichiometric coefficient of each product/reactant but the CollegeBoard omits it from the formula for some unknown reason.
Interpreting The Sign of ΔS°
Another important way of looking at entropy is by observing the sign of ΔS° for a reaction and drawing conclusions as to whether or not the reaction became more or less ordered and vice versa—predicting the sign of ΔS° by looking at properties of a reaction. When ΔS° is positive, a reaction creates disorder and thus increases the entropy of the system. Conversely, when ΔS° is negative, the reaction reduces disorder or in other words creates order. Therefore the entropy of the system decreases.
By using these rules we can also predict the opposite: the sign of ΔS° of a reaction given the reaction. We’ll take a look at a few of these in the practice problems.
Practice Problems
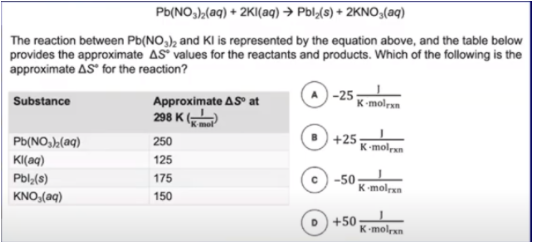
In this problem, we’re given a reaction along with the entropies of various substances at 298K. For this problem we can simply use our equation for S° to find the ΔS° for the reaction:
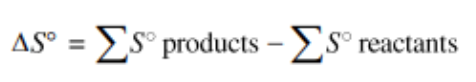
= ((175) + 2(150)) - (250 + 2(125)) = -25 J/molrxnK ⇒ A.
Note that we multiplied 150 and 125 by 2 because the stoichiometric coefficients on KI and KNO3 are 2.
2. Consider the reaction: 2Na (s) + Cl2 (g) → 2NaCl (s). Predict the sign of ΔS° for this reaction and explain
Looking at this reaction, we see two reactants, sodium and chlorine, forming one solid product, sodium chloride. Let’s think about the disorder of the reactants versus the disorder of the products. On the reactants side we have 3 total moles (2 + 1 = 3) of reactants, one of which is a gas. These react to form fewer moles of a solid (less chaotic) product. Therefore, our reaction is more chaotic at the beginning and becomes less chaotic. This means in the process we “lose” entropy (we don’t actually destroy entropy because of the first law of thermodynamics, but we can think of it this way). Therefore, we can predict that ΔS° for this reaction is negative.
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.