Dalia Savy
Jacob Jeffries
AP Chemistry 🧪
269 resourcesSee Units
So far in this unit, we've been discussing the kinetics of only elementary reactions. These occur in a single step and typically involve only a single molecule or a group of atoms. These are the most basic types of chemical reactions and a starting point for understanding more complex reactions.
Now that we've got the basics down, let's get into the kinetics of complex reactions (which are more of what happens in real life).
What is a Mechanism?
Reaction mechanisms go more in-depth regarding chemical reactions than just a net equation. For example, a reaction between an acid and a base must happen in the presence of water, yet water is not included as a reactant. The actual reaction happens in two steps.
Elementary Steps
A mechanism basically takes a full reaction and splits it up into elementary steps. These steps essentially show what actually happens in a reaction. Let's take a look at the reaction mechanism for the decomposition of hydrogen peroxide (H₂O₂):
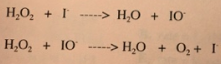
Rather than trying to understand the reaction by looking at the net equation, we can break it down to the level of its elementary steps. You can think of elementary steps as the smallest unit of a reaction that can be studied, as they are simple and individual steps that make up a reaction that result in a certain product.
As we can see, the decomposition of hydrogen peroxide occurs in two elementary steps:
- In the first step, hydrogen peroxide molecules react with iodide to form water molecules and iodite ions. We can see that one of the oxygen atoms from hydrogen peroxide reacts with the iodide to form iodite ions.
- In the next step, we have hydrogen peroxide reacting with the product of the last step: the iodite ions. In this process, another oxygen atom reacts, but with iodite this time, to form more water molecules, oxygen gas, and iodide.
Try not to think of chemical reactions as mathematical equations. Imagine we are completing this reaction in a beaker. We have tons of hydrogen peroxide molecules reacting with tons of iodide ions, which is why hydrogen peroxide can be a reactant in both steps.
When we add up all of the individual elementary steps and cancel out the spectators, we end up with the overall balanced chemical equation (or net equation).
Why is this important, you may ask? Well, if we find the total reaction by adding the elementary steps, we see that IO⁻ and I⁻ cancel out to leave 2H₂O₂ → 2H₂O + O₂. So wait...why are the iodite and iodide ions even there? Let's get into that!
Catalysts and Intermediates
Since elementary steps show what is happening at a molecular level, their components may involve intermediates and catalysts as well as reactants and products.
Iodide as a Catalyst
In the reaction above, iodide acts as a catalyst. We'll discuss how catalysts work in chemical reactions in more depth later in this unit, but let's cover the basics. A catalyst is essentially a substance that increases the rate of a chemical reaction without being consumed in the process. It speeds up the reaction without being affected by it, as it goes in and comes out the exact same way.
Since the catalyst is not in the overall balanced equation, we can see that it does not impact the reaction products, but rather impacts the mechanism by changing the way the reaction occurs. The natural decomposition of H2O2 actually occurs, but it is incredibly slow. In a lab, we can't always wait, so sometimes we use catalysts to speed things up. 🏃
Iodite as an Intermediate
So, iodide is a catalyst, but what is iodite? The iodite ion produced in the first elementary step, and then consumed in the next, is an intermediate. Intermediates are species that are formed during a reaction and then go on to participate in further reactions. They are not reactants or products, but are present in the reaction mixture in significant concentrations only while the reaction itself is occurring.
What a mechanism looks like for a more complex reaction. Luckily you won't be dealing with these! Image Courtesy of ResearchGate
Mechanisms and Rate Laws
Something we haven't discussed yet is that each elementary step has its own rate constant and activation energy. What can this tell us when looking at a reaction mechanism?
Rate-Determining Steps
Often when dealing with mechanisms, you will see one elementary step labeled "slow" and the others labeled "fast" since they have their own respective rate constants. The slow step is also called the rate-determining step as it defines the rate law of the overall reaction. This should make sense! A reaction can only proceed as fast as its slowest step.
Remember: The rate-determining step is the slowest step in a reaction mechanism and controls the overall rate of the reaction. The overall rate of a reaction is equal to the rate of the rate-determining step.
For a conceptual understanding, in a multi-step reaction, the rate-determining step is typically the step that has the highest activation energy. Since the rate of a reaction is directly proportional to the frequency of successful collisions between reactant molecules with enough energy to overcome the activation energy barrier, a step with a higher activation energy will have a lower rate constant and, therefore, will be slower than other steps. Thus, the rate-determining step sets the pace for the entire reaction and all other steps must keep up with it.
Example of Rate-Determining Step
Let's go through this two-step reaction mechanism to better understand this concept and calculate the rate law of the overall reaction.

Example Courtesy of the Organic Chemistry Tutor
The first step to calculating the rate law of the overall reaction is to actually figure out what the overall reaction is. If you add up both elementary steps and then cancel out the intermediate (HI), you should get H₂ + 2ICl → I₂ + 2HCl.
The next step is to figure out which elementary step is the rate-determining step of the reaction. This is pretty easy; just find the elementary step that is slow! In this mechanism, it is the first elementary step.
Now, it is time to calculate the rate law of the first elementary step since it is the rate-determining step. When dealing with elementary steps (and only elementary steps), we can use the stoichiometric coefficients to tell us the order for each reactant. Thus, looking at the slow reaction, we know that the rate law of the overall reaction is: R = k[H₂][ICl]
You can see why this concept can lead to lots of errors. If a student were to forget to use the rate law of the rate-determining step, and rather calculated it by looking at the overall balanced equation, they would have had an exponent of two for ICl.
When the Slow Step Has Intermediates
An important thing to note is that sometimes the rate-determining step will have an intermediate in it. In this case, you will need to use some math to make a substitution since you cannot have an intermediate in your rate law. This math involves a topic in chemistry that you most likely learned called equilibrium. If you have, what you do is you essentially use the Keq of one of the elementary steps (typically one will be in "fast equilibrium") and use some substitutions. To see what this looks like, check out the next study guide!
Example Mechanism
The following mechanism was actually part of the 2019 AP Chemistry examination. Let's try to find the rate law of the overall reaction!
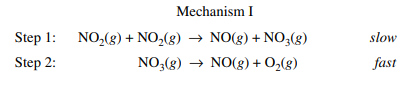
Example Courtesy of College Board
To find the rate law for this mechanism, we look to the slow, rate-limiting step. We find the rate-limiting step to be step one. By using the stoichiometric coefficients (which again, we can ONLY do with elementary steps), we find the rate law to be R = k[NO₂][NO₂], or once you simplify it, R = k[NO₂]².
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
🧐Exam Skills
📚Study Tools

Fiveable
Resources
© 2025 Fiveable Inc. All rights reserved.