AP Chemistry 🧪
269 resourcesSee Units
Multiple Choice Practice for Equilibrium
Welcome to Unit 7 AP Chemistry Multiple Choice Questions! Grab some paper and a pencil 📄 to record your answers as you go. You can see how you did on the Unit 7 Practice Questions Answers and Review sheet once you're done. Don't worry, we have tons of resources available if you get stumped 😕 on a question. And if solo study is not your thing, join a group in Hours!
Not ready to take a quiz yet? Take a look at the Intro to Unit 7

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and, although they cover information outlined in the AP Chemistry Course and Exam Description, the formatting on the exam may be different.
1. Which of the following correctly defines equilibrium?
A. When the rate of the forward reaction is equal to the rate of the reverse reaction.
B. When the concentration of the product is equal to the concentration of the reactant.
C. When the rate of the forward reaction is equal to the concentration of the reactants.
D. When the equilibrium constant, K, is equal to 1.
2. Which of the following is the equilibrium expression for the following reaction?
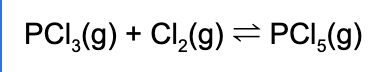
3. Which of the following will increase the solubility of iron (III) hydroxide?
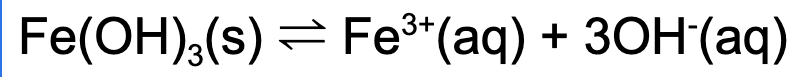
A. Adding a catalyst
B. Adding sodium chloride (NaCl)
C. Adding sodium hydroxide (NaOH)
D. Adding hydrochloric acid (HCl)
4. What is the value for the equilibrium constant, K, given the following data?
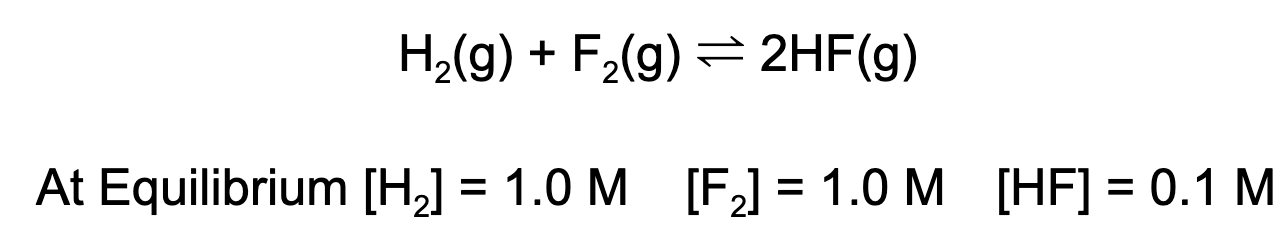
A. 0.01
B. 0.1
C. 10
D. 100
5. A reaction at equilibrium is placed into an oven. Which of the following statements is true?
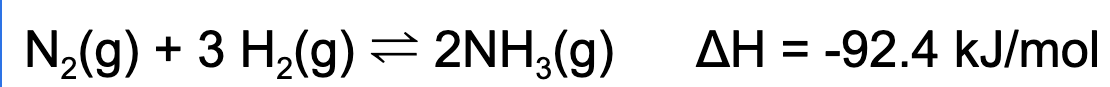
A. The concentration of ammonia gas will increase
B. The concentration of hydrogen gas will increase.
C. The concentration of nitrogen gas will decrease.
D. The concentration of all reactants and products will increase.
6. A reaction vessel contains some amount of calcium carbonate and calcium oxide. If carbon dioxide is injected into the vessel such that it has a partial pressure of 1.0 atm, which of the following is true?
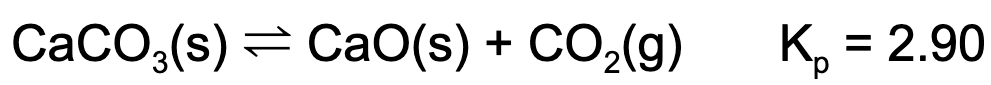
A. The mass of calcium carbonate will decrease.
B. The mass of calcium oxide will decrease.
C. The partial pressure of carbon dioxide will decrease.
D. Nothing, the reaction is at equilibrium.
7. When the temperature of the reaction vessel increased, the solution becomes darker in color. Which of the following is true?
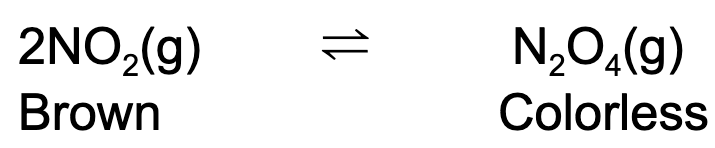
A. The reaction is endothermic. K increases as temperature decreases.
B. The reaction is exothermic. K increases as temperature increases.
C. The reaction is exothermic. K increases as temperature decreases.
D. The reaction is endothermic. K increases as temperature increases.
8. At equilibrium, the concentration of sulfur dioxide and oxygen gas is 1.00 M. What is the concentration of sulfur trioxide at equilibrium?
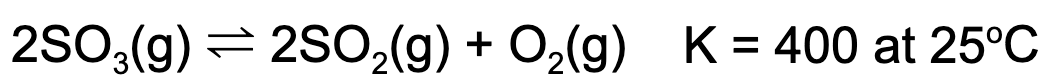
A. 0.0025 M
B. 0.05 M
C. 400 M
D. 20 M
9. A reaction vessel is filled such that the concentration of hydrogen gas, chlorine gas, and hydrogen chloride each have a partial pressure of 1.0 atm. What happens to the total pressure of the reaction vessel as the reaction proceeds to equilibrium?
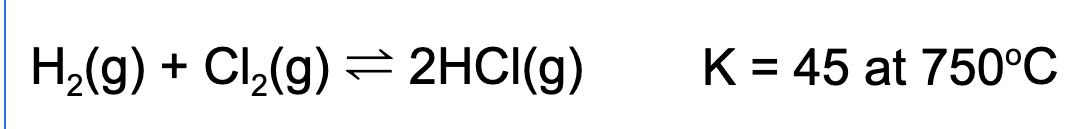
A. The total pressure will increase because Q < K.
B. The total pressure will decreas because Q < K.
C. The total pressure will increase because Q > K.
D. The total pressure will remain constant because there are equal quantities of reactants and products.
10. What is the value of Kp given that, at equilibrium, the partial pressure of oxygen gas is 2.0 atm and the partial pressure of ozone is 4.0 atm at a particular temperature?
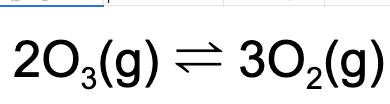
A. 0.50
B. 2.0
C. 8
D. 0.25
11. A 2.0 L vessel is charged with 10 mol of nitrogen monoxide and sufficient chlorine gas. At equilibrium, the concentration of nitrogen monoxide is 3.0 M. What is the concentration of NOCl at equilibrium?
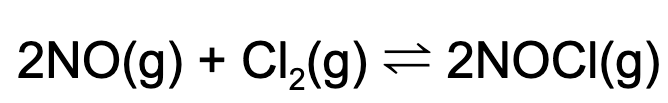
A. 2.0 M
B. 1.0 M
C. 7.0 M
D. 3.5 M
12. What is the definition of the common ion effect?
A. The solubility of a salt in a solution that contains a common ion is greater than in pure water.
B. If the concentration of a common ion is greater than the molarity of the salt dissolving in a solution, then a precipitate will form.
C. The solubility of a salt in a solution that contains a common ion is less than in pure water.
D. If the concentration of a common ion is less than the molarity of the salt dissolving in a solution, then a precipitate will form.
13. The following equilibrium is established in a reaction vessel. What happens when argon (Ar) is added to a reaction vessel?
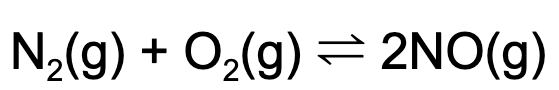
A. The concentration of NO will increase.
B. The partial pressure of oxygen gas will increase.
C. The partial pressure of oxygen gas will decrease.
D. No change will occur.
14. Which of the following will cause an increase in concentration in BrCl(g)?
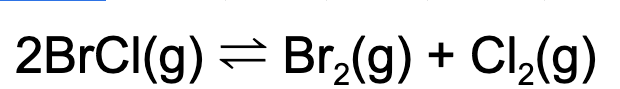
A. Adding a catalyst
B. Adding chlorine gas to the reaction vessel
C. Adding xenon (Xe)
D. Removing bromine gas
15. What is the value for Ksp if the concentration for the chlorine ion is 0.1 M and the concentration of lead is 0.02 M at a particular temperature in a saturated solution?
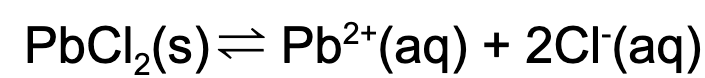
A. 5000
B. 50
C. 0.02
D. 0.0002
- 🙌 Time to check your answers on Unit 7 Practice Questions Answers and Review
- 🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.