AP Chemistry 🧪
269 resourcesSee Units
Multiple Choice Practice for Molecular and Ionic Compound Structures and Properties
Welcome to Unit 2 AP Chemistry Multiple Choice Questions! Grab some paper and a pencil 📄 to record your answers as you go. You can see how you did on the Unit 2 Practice Questions Answers and Review sheet once you're done. Don't worry, we have tons of resources available if you get stumped 😕 on a question. And if solo study is not your thing, join a group in Hours!
Not ready to take a quiz yet? Take a look at the Intro to Unit 2.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and, although they cover information outlined in the AP Chemistry Course and Exam Description, the formatting on the exam may be different.
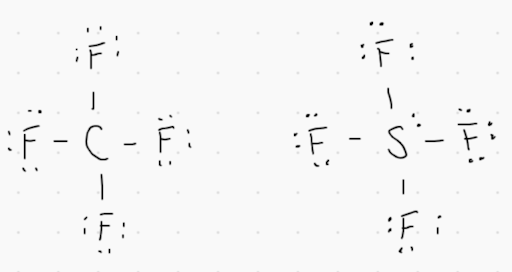
1. Lewis dot diagrams for CF_4 and SF_4 are provided in the image above. Which of the following statements are true?
A. The molecular geometry for both substances is different because S has a greater number of electron domains than C.
B. Both molecules have the same shape because the molecular formulas are the same.
C. Both molecules have similar shapes and are nonpolar because the electronegativities for C and S are similar.
2. Which of the following ionic compounds will have the highest melting point? Predict the relative lattice energies of the salts.
A. NaCl
B. KF
C. K_2O
3. In the molecule COCl_2, how many sigma and pi bonds are present?
A. 2 sigma and 1 pi bond
B. 3 sigma and 1 pi bond
C. 3 sigma bonds only
4.

A. Gold, Au(s)
B. Ammonium Nitrate, NH_4NO_3(s)
C. Ethanol, CH_20H(I)
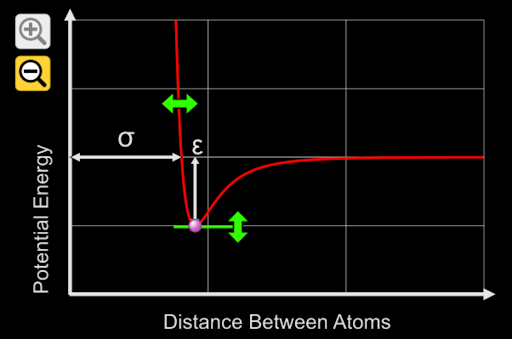
5. A potential energy graph of O_2 is represented in the graph above. Which of the following is the potential energy on the y-axis representing?
A. Bond Length
B. Electronegativity
C. Bond Energy
D. Type of Bond
6. PCl_3 is polar and PF_5 is nonpolar. Which of the following statements correctly justifies the difference in polarity?
A. The P--F bonds are nonpolar while the P--Cl bonds are polar
B. PF_5 has a symmetrical geometry, whereas PCl_3 has a nonsymmetrical geometry.
C. P--F is a single bond while P--Cl is a double bond.
7. Which of the following descriptions seems most appropriate for the substance CBr_4?
A. CBr_4 is an electrolyte.
B. CBr_ 4 is a solid at room temperature and is hard, but brittle.
C. CBr_4 is malleable, ductile, and lustrous.
8. What is the molecular geometry of formaldehyde, CH_2O?
A. Trigonal Pyramidal
B. Trigonal Planar
C. Tetrahedral
9. Which of the following descriptions best fits the substance molybdenum, Mo?
A. Mo is malleable, ductile, and lustrous.
B. Mo is a gas at room temperature.
C. Mo is a solid at room temperature and is hard, but brittle.
10. Which of the following molecules has a resonance structure?
A. NH_3
B.CCI_4
C.SF_2
11. What is the hybridization for the SO_3
A. sp
B. sp^2
C. sp^3
12. Which of the following is a rigid alloy?
A. Steel (Fe & C)
B. Brass (Cu & Zn)
C. Sodium chloride (NaCl)
13. Which of the following is the formula for the compound formed by aluminum and chlorine atoms?
A. Al_3Cl
B. AlCl
C. AlCl_2
14. Which of the following substances conducts electricity in the solid phase?
A. Diamond (C)
B. Ethanol (CH_3OH)
C. Zinc (Zn)
15. A substance that is composed of only nonmetals but has a very high melting point is called....
A. Network covalent solid
B. Ionic Solid
C. Molecular Solid
D. Metallic Solid
- 🙌 Time to check your answers on Unit 2 Practice Questions Answers and Review.
- 🤝Connect with other students studying AP Chem with Hours!
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.