AP Chemistry 🧪
269 resourcesSee Units
Answers and Review for Multiple Choice Practice on Molecular and Ionic Compound Structures and Properties
⛔STOP ! ⛔ Before you look at the answers, make sure you gave this practice quiz a try so you can assess your understanding of the concepts covered in Unit 2. Click here for the practice questions: AP Chemistry Unit 2 Multiple Choice Questions.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and although they cover information outlined in the AP Chemistry Course and Exam Description the formatting on the exam may be different.
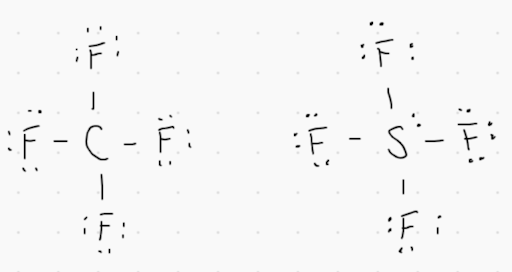
1. Lewis dot diagrams for CF_4 and SF_4 are provided in the image above. Which of the following statements are true?
A. The molecular geometry for both substances is different because S has a greater number of electron domains than C.
B. Both molecules have the same shape because the molecular formulas are the same.
C. Both molecules have similar shapes and are nonpolar because the electronegativities for C and S are similar.
D. The lone pairs on S make is the negative end of the dipole.
Answer: An electron domain is a lone pair or a bonding group. The two molecules have different shapes because the total number of electron domains are different.
📄 Study AP Chem, Unit 2.5: Lewis Dot Diagrams
2. Which of the following ionic compounds will have the highest melting point? Predict the relative lattice energies of the salts.
A. NaCl
B. KF
C. K_2O
D. CaO
Answer: The lattice energy for CaO is greatest because the magnitude of charge difference is greatest between the Ca^2+ ions and the O^2- ions. As lattice energy increases, the melting point of the ionic compound increases.
📄 Study AP Chem, Unit 2.3: Ionic Bonding and Ionic Solids
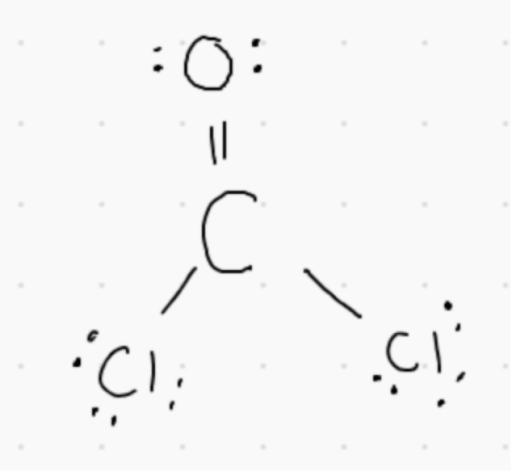
3. In the molecule COCl_2, how many sigma and pi bonds are present?
A. 2 sigma and 1 pi bond
B. 3 sigma and 1 pi bond
C. 3 sigma bonds only
D.3 pi bonds only
Answer: In the Lewis structure, there are 2 single bonds and 1 double bond. For every single bond there is 1 sigma bond. For every double bond, there is 1 sigma bond and 1 pi bond.
📄 Study AP Chem, Unit 2.7: VSEPR, Bond Hybridization, and Molecular Geometry
4.

A. Gold, Au(s)
B. Ammonium Nitrate, NH_4NO_3(s)
C. Ethanol, CH_20H(I)
D. Potassium Chloride, KCl(s)
Answer: Covalent compounds have relatively low melting and boiling points compared to ionic compounds and metallic solids. The only covalent compound is ethanol.
📄 Study AP Chem, Unit 2.4: Metallic Bonding and Alloys
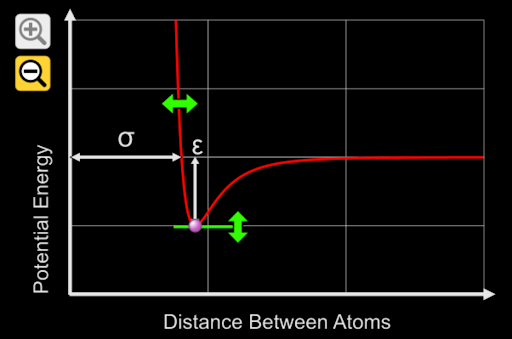
5. A potential energy graph of O_2 is represented in the graph above. Which of the following is the potential energy on the y-axis representing?
A. Bond Length
B. Electronegativity
C. Bond Energy
D. Type of Bond
Answer: The potential energy on the y-axis measures the bond energy, the amount of energy required to separate the two oxygen atoms.
📄 Study AP Chem, Unit 2.2: Intramolecular Force and Potential Energy
6. PCl_3 is polar and PF_5 is nonpolar. Which of the following statements correctly justifies the difference in polarity?
A. The P--F bonds are nonpolar while the P--Cl bonds are polar
B. PF_5 has a symmetrical geometry, whereas PCl_3 has a nonsymmetrical geometry.
C. P--F is a single bond while P--Cl is a double bond.
D. P--Cl is an ionic bond while P--F is a covalent bond.
Answer: Symmetrical molecules are nonpolar regardless of the type of bonds. Unsymmetrical molecules, like PCl_3, are polar if the bonds have a significant electronegativity difference.
7. Which of the following descriptions seems most appropriate for the substance CBr_4?
A. CBr_4 is an electrolyte.
B. CBr_ 4 is a solid at room temperature and is hard, but brittle.
C. CBr_4 is malleable, ductile, and lustrous.
D. CBr_4 has a boiling point of 97C and cannot conduct electricity.
Answer: CBr_4 is a covalent molecule; therefore, it has a relatively low boiling point and cannot conduct electricity.
📄 Study AP Chem, Unit 2.1: Types of Chemical Bonds
8. What is the molecular geometry of formaldehyde, CH_2O?
A. Trigonal Pyramidal
B. Trigonal Planar
C. Tetrahedral
D. Linear
Answer: Formaldehyde's Lewis structure contains three bonding groups and no lone pairs. This is consistent with trigonal planar molecular geometry.
📄 Study AP Chem, Unit 2.7: VSEPR, Bond Hybridization, and Molecular Geometry
9. Which of the following descriptions best fits the substance molybdenum, Mo?
A. Mo is malleable, ductile, and lustrous.
B. Mo is a gas at room temperature.
C. Mo is a solid at room temperature and is hard, but brittle.
D. Mo has a boiling point of 97C and cannot conduct electricity.
Answer: Molybdenum is a metal. Metals are located on the left side of the periodic table and tend to be malleable, ductile, and lustrous.
📄 Study AP Chem, Unit 2.4: Metallic Bonding and Alloys
10. Which of the following molecules has a resonance structure?
A. NH_3
B.CCI_4
C.SF_2
D. SO_2
Answer: SO_2 is the only molecule in the list that has a resonance structure. Remember resonance structures are when there is more than one appropriate Lewis structure.
📄 Study AP Chem, Unit 2.6: Resonance and Formal Charge
11. What is the hybridization for the SO_3
A. sp
B. sp^2
C. sp^3
D. SO_3 does not have hybridization because it has a resonance structure.
Answer: The hybridization for SO_3 is sp^2 because the molecule has three electron domains. The completed Lewis structure contains three bonds to oxygen and no lone pairs.
📄 Study AP Chem, Unit 2.7: VSEPR, Bond Hybridization, and Molecular Geometry
12. Which of the following is a rigid alloy?
A. Steel (Fe & C)
B. Brass (Cu & Zn)
C. Sodium chloride (NaCl)
D. Dry Ice (CO_2)
Answer: Steel is an interstitial alloy. Interstitial alloys are more rigid than pure metals and substitutional alloys because the smaller atoms fit in the holes created by the parent atoms. This makes it difficult for the host atom to move around.
📄 Study AP Chem, Unit 2.4: Metallic Bonding and Alloys
13. Which of the following is the formula for the compound formed by aluminum and chlorine atoms?
A. Al_3Cl
B. AlCl
C. AlCl_2
D. AlCl_3
Answer: When aluminum is in an ionic compound, it has a charge of 3+. When chlorine is in an ionic compound, it has a charge of 1-. Therefore the ratio that elements come in to make a neutral compound is 1 Al: to 3 Cl ions.
📄 Study AP Chem, Unit 2.3: Ionic Bonding and Ionic Solids
14. Which of the following substances conducts electricity in the solid phase?
A. Diamond (C)
B. Ethanol (CH_3OH)
C. Zinc (Zn)
D. Potassium bromide (KBr)
Answer: Zinc is a metal. Metals are the only substance that conduct electricity in the solid phase.
📄 Study AP Chem, Unit 2.7: VSEPR, Bond Hybridization, and Molecular Geometry
15. A substance that is composed of only nonmetals but has a very high melting point is called....
A. Network covalent solid
B. Ionic Solid
C. Molecular Solid
D. Metallic Solid
Answer: Network covalent solids are giant molecules. In order to melt them, the bonds between the atoms must be broken, which requires more energy than overcoming intermolecular forces.
📄 Study AP Chem, Unit 2.4: Metallic Bonding and Alloys
What can we help you do now?
🔍Check out all of the resources for AP Chem Unit 3
🦘Jump to AP Chem Unit 3 Multiple Choice Questions
🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.