AP Chemistry 🧪
269 resourcesSee Units
Answers and Review for Multiple Choice Practice on Thermochemistry and Reaction Thermodynamics
⛔STOP ! ⛔ Before you look at the answers, make sure you gave this practice quiz a try so you can assess your understanding of the concepts covered in Unit 6. Click here for the practice questions: AP Chemistry Unit 6 Multiple Choice Questions.

Image courtesy of Pixabay
Facts about the test: The AP Chemistry exam has 60 multiple choice questions and you will be given 1 hour 30 minutes to complete the section. That means it should take you around 15 minutes to complete 10 questions.
*The following questions were not written by College Board and although they cover information outlined in the AP Chemistry Course and Exam Description the formatting on the exam may be different.
1. Which of the following is defined by the measure of the average kinetic energy?
A. Potential Energy
B. Velocity
C. Heat
D. Temperature
Explanation: Temperature is defined as the measure of the average kinetic energy of the sample.
Read this overview of Unit 6!
2. What is the amount of energy is required to raise the temperature of 43 g of liquid water from 25°C to 55°C? Water's specific heat is 4.18 J/g°C.
A. 5.39 kJ
B. 5390 kJ
C. 4.49 kJ
D. 9.88 kJ
Explanation: Use the equation Q=mCΔT. The change in temperature (ΔT) is 30°C, the mass is 43 g, and the specific heat of liquid water is 4.18 J/g°C. There are 1000J for every kJ. Therefore, the hear is 5.39 kJ.
Read this guide about heat capacity!
3. What is the amount of energy required to melt 7.2 g of water at 0°C? Water's enthalpy of fusion is 6.01 kJ/mol and water's enthalpy of vaporization is 40.7 kJ/mol.
A. 16.3 kJ
B. 43.3 kJ
C. 293 kJ
D. 2.40 kJ
Explanation: We must use water's enthalpy of fusion because that refers to the amount of energy required to melt 1 mol of water. The heat of vaporization refers to vaporizing 1 mol of water.
Read this guide about enthalpies!
4. When this reaction occurs, 75.0 kJ of energy is released. What mass of copper is produced?
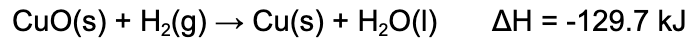
A. 0.588 g
B. 36.7 g
C. 110 g
D. 1.73 g
Explanation: 1 mol of copper is produced for every 129.7 kJ of energy that is released by this reaction. Using this as a conversion factor, one finds that 0.588 mol of copper should be produced. Multiply by 63.55 g/mol, copper's molar mass, to find 36.7 g of copper is produced.
Read this guide about heat capacity!
5. Which of the following statements is true regarding the following reaction?
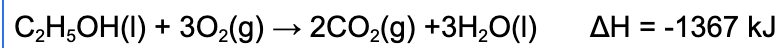
A. The reaction will cause the surrounding's temperature to increase because the reaction is exothermic.
B. The reaction will cause the surrounding's temperature to decrease because the reaction is exothermic.
C. The reaction will cause the surrounding's temperature to increase because the reaction is endothermic.
D. The reaction will cause the surrounding's temperature to decrease because the reaction is endothermic.
Explanation: When enthalpy (ΔH) is negative, this indicates the reaction is exothermic. Therefore, the reaction will release energy to the surrounding causing the temperature to increase.
Read this guide about exothermic vs. endothermic reaction!
6. Determine the enthalpy of the reaction given the following data.
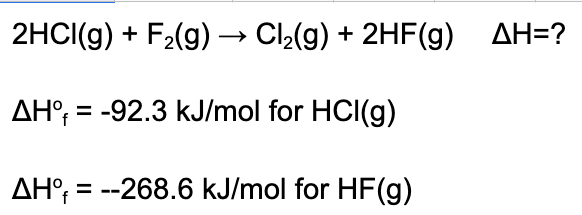
A. 352.6 kJ/mol
B. -352.6 kJ/mol
C. 176.3 kJ/mol
D. -176.3 kJ/mol
Explanation: Given the heats of formation we can use the "products minus reactants" equation. ΔH = [2x-268.6]-[2x-92.3]
Read this guide introducing enthalpies of reaction!
7. Which of the following is an endothermic process?
A. Combusting methane
B. Freezing benzene
C. Condensing water
D. Melting wax
Explanation: Consider the relative energy of the states of matter. Since solid has less energy than liquid, melting is the only endothermic process listed in the options.
Read this guide about exothermic vs. endothermic reaction!
8. What is the enthalpy of reaction depicted in the reaction below?
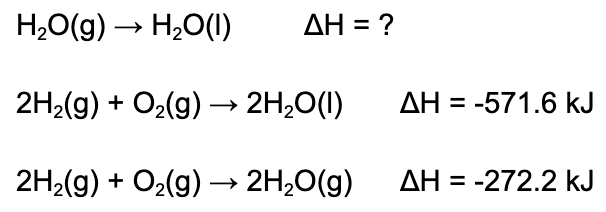
A. -299.4 kJ/mol
B. 149.7 kJ/mol
C. -149.7 kJ/mol
D. 299.4 kJ/mol
Explanation: In order to determine the enthalpy of reaction, one must use Hess's Law. Both reactions with given enthalpies must be halved. The second reaction also must be flipped. Once this is done, add up the enthalpies to determine the new one.
Read this guide introducing enthalpies of reaction!
9. Which of the following process is exothermic?
A. CH4 (g) + 2O2(g) -> 2H2O(g) + CO2 (g)
B. H2O (l) -> H2O (g)
C. CO2 (s) -> CO2 (g)
D. CaCO3 (s) -> CaO (s) + CO2(g)
Explanation: All combustion reactions are exothermic. Decomposition reactions are endothermic. Melting, evaporating, and sublimation are endothermic.
Read this guide about exothermic vs. endothermic reaction!
10. When hot piece of metal is placed into cold water, which of the following statements is true?
A. The final temperature for the metal will be hotter than the initial temperature of the metal at thermal equilibrium.
B. The final temperature of the metal will be cooler than the initial temperature of the water at thermal equilibrium.
C. The average kinetic energy of the water will increase while the average kinetic energy for the metal will decrease.
D. The average kinetic energy of the metal will increase while the average kinetic energy for the water will decrease.
Explanation: Heat transfer always occurs from hot to cold. The metal transfers its energy into water heating it up while cooling the metal down. The final temperature will be the same at thermal equilibrium.
Read this guide about heat transfer!
11. A 25 g piece of metal required 227.5 J of energy to raise it by 10°C. What is the identity of the metal?
A. Iron (C=0.44 J/g°C)
B. Aluminum (C=0.91 J/g°C)
C. Copper (C=0.39 J/g°C)
D. Lead (C=0.13 J/g°C)
Explanation: This question is asking you to determine the specific heat. For a metal, the specific heat of the substance is an intensive property, meaning it doesn't change regardless the amount of matter one has. Therefore, by using Q=mCΔT, one finds the identity of the metal.
Read this guide about heat capacity!
12. How much heat is released when 5.00 g of propane (M=44.11g/mol) combusts? The heat of combustion for propane is -2220 kJ/mol.
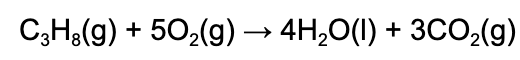
A. 11100 kJ
B. 252 kJ
C. 50.3 kJ
D. 84.0 kJ
Explanation: 5.00 g of propane is 0.113 mol of propane. Using the enthalpy as a conversion factor, one finds that when 1 mol of propane combusts, 2220 kJ of energy is released.
Read this guide about energy of phase changes!
13. Consider the following reaction. Which of the following statements is true?
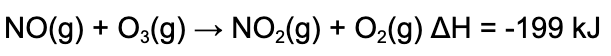
A. More energy is released when the bonds form than energy is required to break the bonds, because the reaction is exothermic.
B. More energy is released when the bonds form than energy is required to break the bonds, because the reaction is endothermic.
C. Less energy is released when the bonds form than energy is required to break the bonds, because the reaction is exothermic.
D. Less energy is released when the bonds form than energy is required to break the bonds, because the reaction is endothermic.
Explanation: Using Q=mCΔT, one finds the change of temperature to be 36°C. Add the initial temperature to find the final temperature of 51°C.
Read this guide about exothermic vs. endothermic reaction!
14. What is the final temperature of 15 g of nickel (C=0.44 J/g°C) that absorbs 240 J of energy at 15°C?
A. 36°C
B. -21°C
C. 22°C
D. 51°C
Explanation: Using Q=mCΔT, one finds the change of temperature to be 36°C. Add the initial temperature to find the final temperature of 51°C.
Read this guide about heat capacity!
15. A student uses a calorimeter to determine the enthalpy of combustion of methane. Which of the following statements is true?
A. After the combustion the temperature of the calorimeter will decrease because combustion reactions are exothermic.
B. After the combustion the temperature of the calorimeter will increase because combustion reactions are exothermic.
C. After the combustion the temperature of the calorimeter will increase because combustion reactions are endothermic.
D. After the combustion the temperature of the calorimeter will decrease because combustion reactions are endothermic.
Explanation: All combustion reactions are exothermic. This causes the surrounding, the calorimeter, to increase in temperature.
Read this guide about exothermic vs. endothermic reaction!
What can we help you do now?
- 🔍Check out all of the resources for AP Chem Unit 7
- 🦘Jump to AP Chem Unit 7 Multiple Choice Questions
- 🤝Connect with other students studying AP Chem with Hours
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

Fiveable
Resources
© 2023 Fiveable Inc. All rights reserved.